SAP Teched 2018 in Barcelona/Spain

We visited this year's SAP Teched in Barcelona and learned great news about the upcoming and already existing SAP technologies. As usual, TechEd was super planned by SAP. The topic…

We visited this year's SAP Teched in Barcelona and learned great news about the upcoming and already existing SAP technologies. As usual, TechEd was super planned by SAP. The topic…

With the adoption of the new EU Medical Device Regulation (MDR) by the European Parliament on April 5, 2017, the European UDI Regulation on the Labelling and Registration of Medical…

Our GS1 barcode generator for SAP forms is already successfully used by many customers. The fully integrated SAP solution enables customers and internal developers to easily and reliably integrate the…

The FDA has laid the foundation with its UDI system and the GUDID database. Many countries are not yet ready to allow medical device manufacturers to automatically upload their data.…
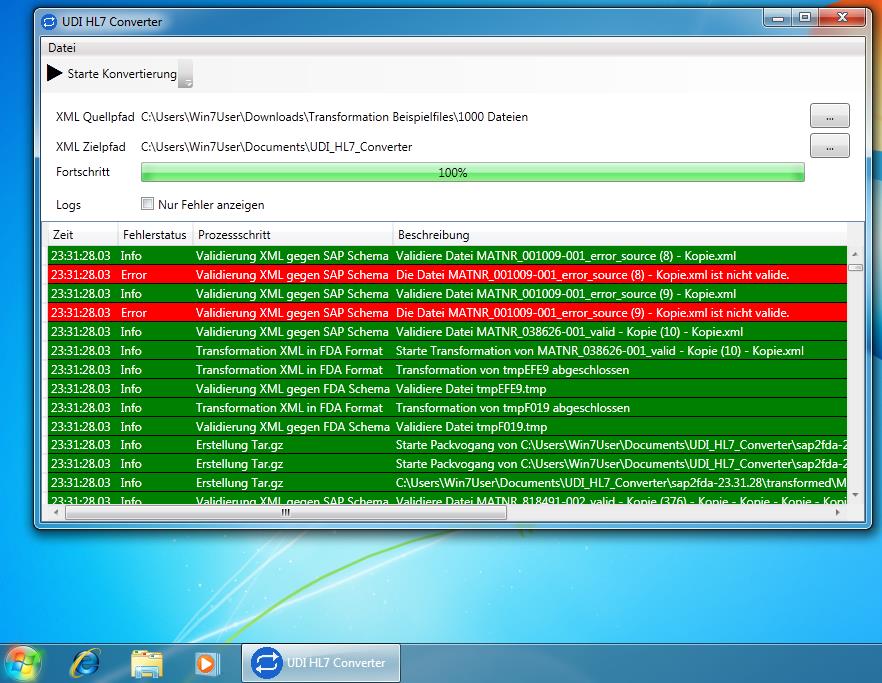
Watch our short presentation video how to configure the UDI add-on, collect data and convert it to HL7 SPL. Watch the video here: UDI Module as SAP Add-Onhttps://www.youtube.com/watch?v=FthEe0N4MyY&feature=youtu.be