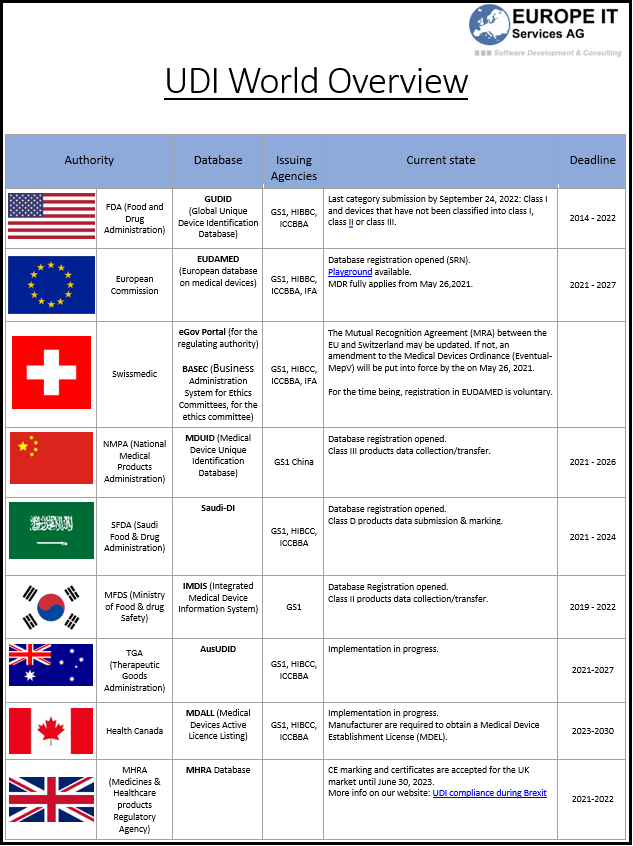
Transitional provisions for the certification of in vitro diagnostic medical devices class D

The IVDR provisions must be applied before May 26, 2022 (during the transition period). As obscurity still remains, the Medical Device Coordination Group (MDCG) recently published indications on how to deal…