New nomenclature for EUDAMED: EMDN
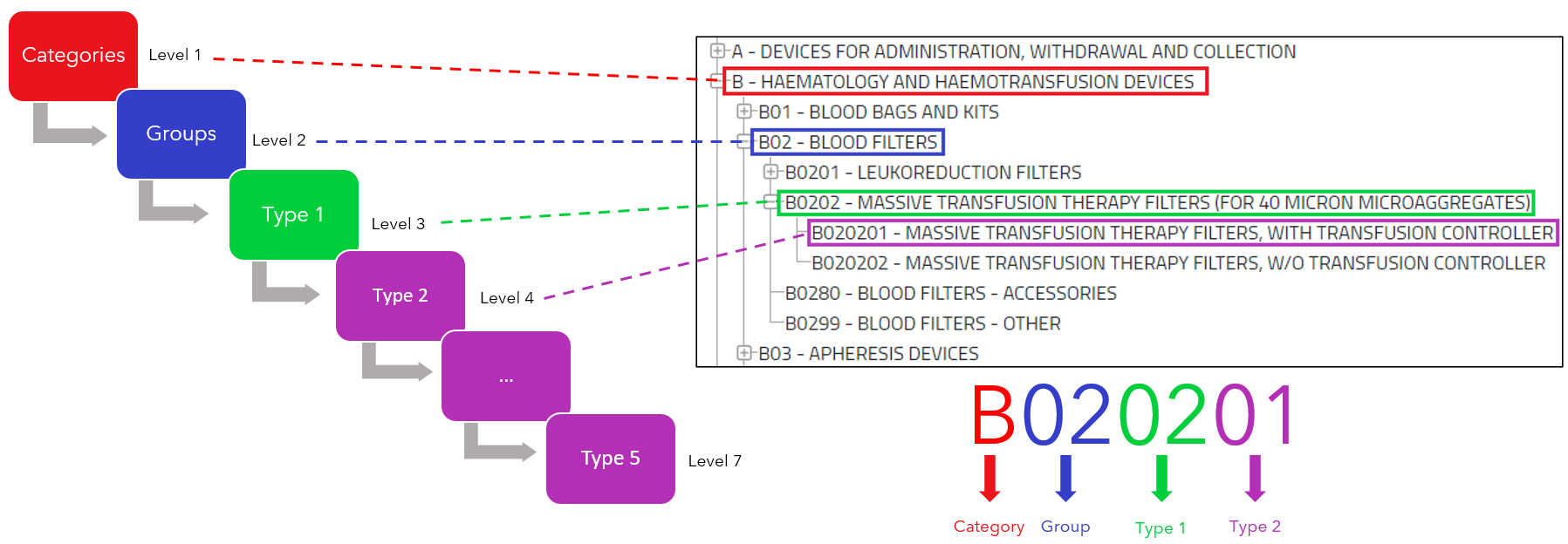
With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept…

With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept…

With the entry into force of MDR 2017/745 on 26.05.2021, the agreement on mutual recognition of medical devices between Switzerland and the EU lost its validity. Thus, Switzerland is now…