Introduction of the UDI system worldwide poses challenges for manufacturers to comply with Unique Device Identification regulations. These obligations are multifaceted, and the path to compliance is highly complex. UDI requirements vary from authority to authority, posing a continuous challenge for companies.
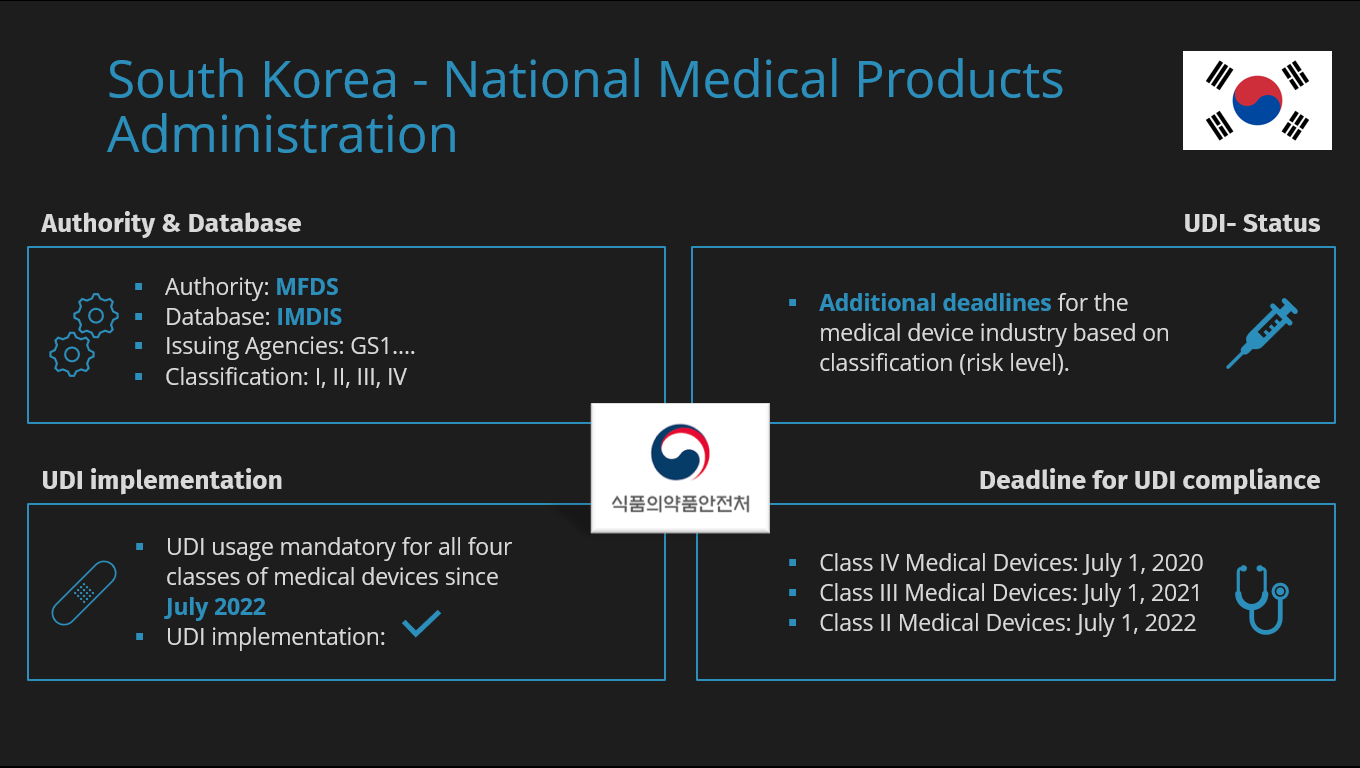
IMDIS: MFDS Database
|
MFDS |
|
|---|---|
| Name of the MFDS UDI Database | IMDIS |
| Link to the MFDS UDI Database | https://emedi.mfds.go.kr |
| Current Status of the IMDIS Database | Live |
UDI Timeline
- Class IV medical devices: July 1, 2020
- Class III medical devices: July 1, 2021
- Class II medical devices: July 1, 2022
- Class I medical devices: July 1, 2023
IMPORTANT:
- A separate Supply Reports (Track & Trace) must be introduced, to be submitted monthly.
- A local representative, “South Korea License Holder”, must be appointed who is responsible for creating the IMDIS account and carries the regulatory responsibilities on behalf of the manufacturer.
If you want to stay updated on regulations and deadlines worldwide, Europe IT Consulting provides global insights available to you.
Technical Requirements and Data Fields of IMDIS
Europe IT Consulting GmbH has created the following table to give you an overview of the technical requirements for the UDI process.
| Technical Requirements and Data Fields |
|---|
| 1. Identification |
| – Primary Device ID |
| – Product/Model Name |
| – Software Version |
| – Barcode Labeling System |
| – Additional Information (URL) |
| 2. Manufacturer/Importer |
| – Manufacturer/Importer License Number |
| – Manufacturer/Importer Name |
| – Manufacturer/Importer Address |
| – Consumer Support Center Name |
| – Consumer Support Center Phone |
| – Consignment Company Name/Address |
| – Foreign Manufacturer Name/Address |
| 3. Regulation |
| – Article Name |
| – Class |
| – Approval, Certification, or Reporting Number of the Article (Article Group) |
| – Date of Approval, Certification, or Reporting |
| – Tracking & Control |
| – Integrated Information Officer’s Phone |
| – Integrated Information Officer’s Email |
| – Health Services under Article 41(3) of the National Health Insurance Act |
| – Health Service Code |
| 4. Safety Information |
| – Contains Latex |
| – Contains Phthalates |
| – MRI Safety Status |
| – Sterile Device |
| – Sterile Required |
| – Sterilization Method |
| – Other Sterilization Methods |
| 5. Packaging |
| – Number of Devices |
| – Package DI |
| – Kit: Respective Name & Class |
| – Combined: Respective Name & Class |
| 6. Characteristics |
| – Implantable |
| – Disposable |
| – Storage Method |
| – Distribution & Handling Conditions |
| 7. Production Control |
| – Lot/Batch Number |
| – Serial Number |
| – Manufacturing Date |
| – Expiry Date |
Europe IT Consulting GmbH’s Solution
Our Global Unique Device Identification (UDI) solution also provides effective data transmission to MFDS. With our offering, we can help you successfully overcome these challenges while optimizing your business processes.
Utilizing our experience and expertise from numerous SAP development projects, we can find and implement an individual and optimal solution for your company.
Our solution supports you in maintaining your UDI-relevant products in SAP and transferring them to medical device databases.
For more information about UDI :
Are you interested?









Related Posts