
GUDID / EUDAMED
What already is true for food today is also becoming reality for medical products. Traceability must be ensured, with the overriding goal of optimizing patient safety. Preparations at medical device manufacturers should be in full swing and there is an urgent need for action. There is not much time left, but it is not too late.
At the heart of this change are the databases of the Food and Drug Administration (FDA) called GUDID (Global Unique Device Identification Database) and those of the EU called EUDAMED (European Database on Medical Devices). Both require a unique product identification, which is handled via the UDI (Unique Device Identification) system. All medical devices must be marked with this unique UDI product identification and transferred to the corresponding database.
The registration of medical devices or the transfer of medical device data to the databases is a step-by-step process based on their risk class and is in its final phase at the FDA. After the successful completion of class 3, 2a and 2b products, the final phase is now underway. This consists of the medical devices of class 1 as well as all non-classified medical devices, which must be transferred to GUDID by 24.09.2020.
In contrast to the FDA, the EU is lagging somewhat behind with the introduction of UDI. The legislation on which the registration of medical devices is based has caused more work than planned. Accordingly, it was published later than planned. In addition, the start of the application of the Medical Device Regulation (MDR 2017/745) has been postponed by one year to May 26, 2021 due to the corona crisis.
The European Parliament adopted the Commission proposal on April 17, 2020. After approval by the member states, the amendment was published in the Official Journal (here for reference) on 23.04.2020 and thus became legally binding. The planned start of the application of the Regulation on in vitro diagnostic medical devices (IVDR 2017/746), which is scheduled for 26.05.2022, is not affected by a postponement and should take place as planned.
Although the prerequisites, such as the completion of EUDAMED, have not yet all been met, companies must prepare themselves in a timely manner. The changeover from MDR or the introduction of UDI has far-reaching consequences. Among other things, the classification, technical documentation, clinical data and the quality management system are affected.
Specificity of the EU UDI data structure
The UDI module of the EU will contain partly the same or similar UDI attributes, as well as new, more detailed information than the comparable database (GUDID) of the American health authority (FDA).
The main difference to GUDID is that the UDI data is divided into the BASIC UDI-DI and the UDI-DI. The BASIC UDI-DI is used to map all common characteristics of a product group. The UDI-DI only contains the product-specific information. There can be several UDI-DIs for a BASIC UDI-DI. Conversely, a UDI-DI is assigned to exactly one BASIC UDI-DI.
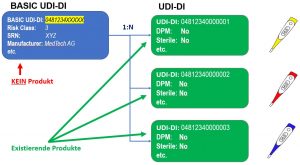
Basic UDI-DI and UDI-DI
As an economic actor, you are responsible for managing all UDI attributes in your own company and transferring the data to EUDAMED.
UDI data transfer to GUDID and EUDAMED
One of the challenges in implementing the requirements of the FDA or the European Commission is to transmit the UDI data to the authorities in the correct format. As a technology company, Europe IT Consulting GmbH offers a service in which the UDI data is transformed into the appropriate format and then transmitted to the authorities.
What possibilities do I have as a customer:
- send UDI data as excel file
You have the possibility to send us your UDI data in an Excel file. For this purpose, we will provide you with an Excel template in which you can maintain your data. We transform the data into the appropriate format and upload it to the database for you. For the transfer of the data to the GUDID, the data is transformed into the required HL7 SPL XML format.
- data transfer with XML files from SAP
Are you already using our UDI SAP Add-On to manage your medical device master data? Then you can either use the data export report to export the data to your local PC and mail the XML files to us or store them directly on our cloud server.
We convert the XML files into the HL7 SPL XML format before transferring the data to GUDID.
- data transfer with the UDI SAP Add-On directly from SAP
If you are already using our UDI SAP Add-On to manage your medical device master data, you can use the data export and simultaneous upload to our cloud server directly from your ERP system.
Through a RESTFull WebService you receive direct confirmation of the successful upload to our Cloud Server.
The UDI add-on then automatically queries the status of the data transfer and the status on the authorities database in the background, so that you are always up to date.
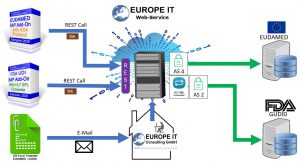
Data transmission to EUDAMED and GUDID








Related Posts