
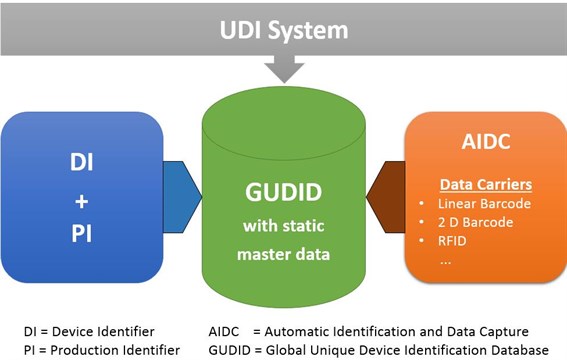
The process of UDI implementation according to EUDAMED and/or FDA requirements is time-consuming and costly. However, there are significant benefits that go far beyond mere compliance. As a manufacturer and/or business actor, you will benefit in the long run once the system is implemented correctly.
As a partner in this process towards UDI compliance, we want to show you the long-term benefits that UDI brings:
- Cost reduction through inventory control
- Due to the high UDI monitoring requirements, your controlling & monitoring process will improve significantly and become more effective.
- Ensure patient safety
- Patients can educate themselves about all products.
- Stricter controls for high risk products.
- Simplified administrative procedures
- Instead of registering products separately in each country, registration of medical devices and actors, e.g. in Europe, only needs to be done once at EU level.
- Transparency guaranteed and equal
- The same rules apply to all business actors, meaning your competitors must publish the same data as you.
- Significant reduction in the number of counterfeit products
- Registration and labeling makes it more difficult for counterfeit products to circulate.
- Valuable help in the event of a merger or acquisition of companies
- The use of UDI data facilitates the checks that need to be carried out before this type of deal between two companies.
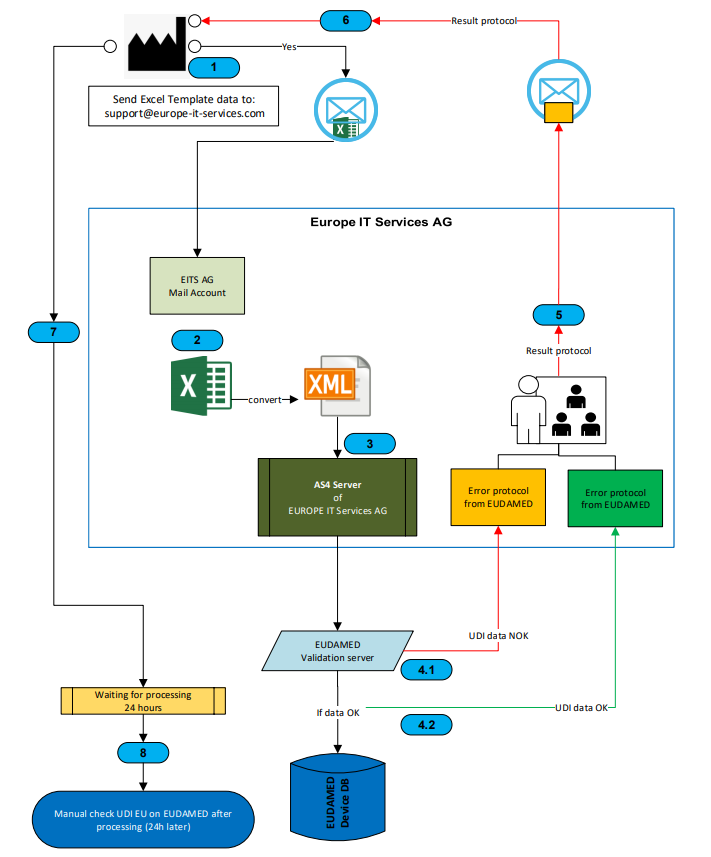
To help you take full advantage of these benefits, we offer SAP solutions that facilitate your UDI implementation and UDI data management. They include all functions from UDI master data creation, UDI master data maintenance to data transfer to EUDAMED or FDA. You can find out more here:







Related Posts