
Which details of the critical warnings have to be provided to EUDAMED, to the GUDID ? The Label information, the instructions for use, or both ? What about the contra indications ?
→ To help you make the right decisions and actions, we provide you with clear and regulatory information about the Storage and Handling conditions and the Critical Warnings, for EUDAMED and GUDID.
For EUDAMED:
Storage & handling conditions
Definition (according to the UDIWG 2018-1): “Indicates storage and handling requirements that are required for the device in accordance with Annex I, 23.2 (k) of the MDR and Annex I, 20.2 (k) of the IVDR.“
For example: “do not cut”, “store in a closed container”, “avoid contact with water”.
Critical Warnings
Definition (according to the Medical device Regulation 2017/745, Annex I, 23.2 about the information the label shall bear) : “Warnings or precautions to be taken that need to be brought to the immediate attention of the user of the device, and to any other person. This information may be kept to a minimum in which case more detailed information shall appear in the instructions for use, taking into account the intended users;”
For example: ” if swallowed: get immediate medical advice/attention”, “explosive when dry”, “toxic by eye contact”.
Following points are to be considered:
- If applicable, these data elements must be entered in EUDAMED and must appear on the label.
- In EUDAMED, and in our Add-On and Excel Templates, both elements are presented as a drop-down list of nomenclature codes with description. So you just have to select the right elements.
- “Only storage/handling conditions and critical warnings or contra-indications, that are required to be on the label, shall be transmitted to the UDI database.” (MDCG 2018-7)
- The conditions and critical warnings “should be provided in English as well as in the languages of those countries where the device is made available.” (MDCG 2018-7)
At the moment, the terms/description associated with the nomenclature codes are only available in English. Therefore, the conditions and critical warnings are to be written manually in the languages of the countries where the device is made available. See our example in SAP below. - A new UDI-DI shall be required whenever there is a change in these data elements. (Medical device Regulation 2017/745, Annex VI, 3.9)
Here is how you can maintain these data elements in our SAP Add-On solution for EUDAMED, by choosing the right category using the drop-down list:

Enter Storage and Handling Conditions in the SAP Add-On for EUDAMED

Enter Critical Warning in the SAP Add-On for EUDAMED
For the FDA:
Critical warnings data are not required in the GUDID.
The conditions to store and handle medical devices, called “Storage and handling Types”, are optional and publicly released if given.
Definition: “requirements that are required for the device including temperature, humidity, and atmospheric pressure“. (GUDID Data Elements Reference Table, 27th April 2020)
If you enter a type of requirement, then its unit of measure and the low or high value must be given as well. Special storage conditions can be entered manually for any special storage requirements.
Note that there can be several types per device record. See our example in SAP below.
Here is how you can maintain the Storage and handling types in our SAP Add-On solution for the FDA:

Enter Storage and Handling Types in the SAP Add-On for the FDA





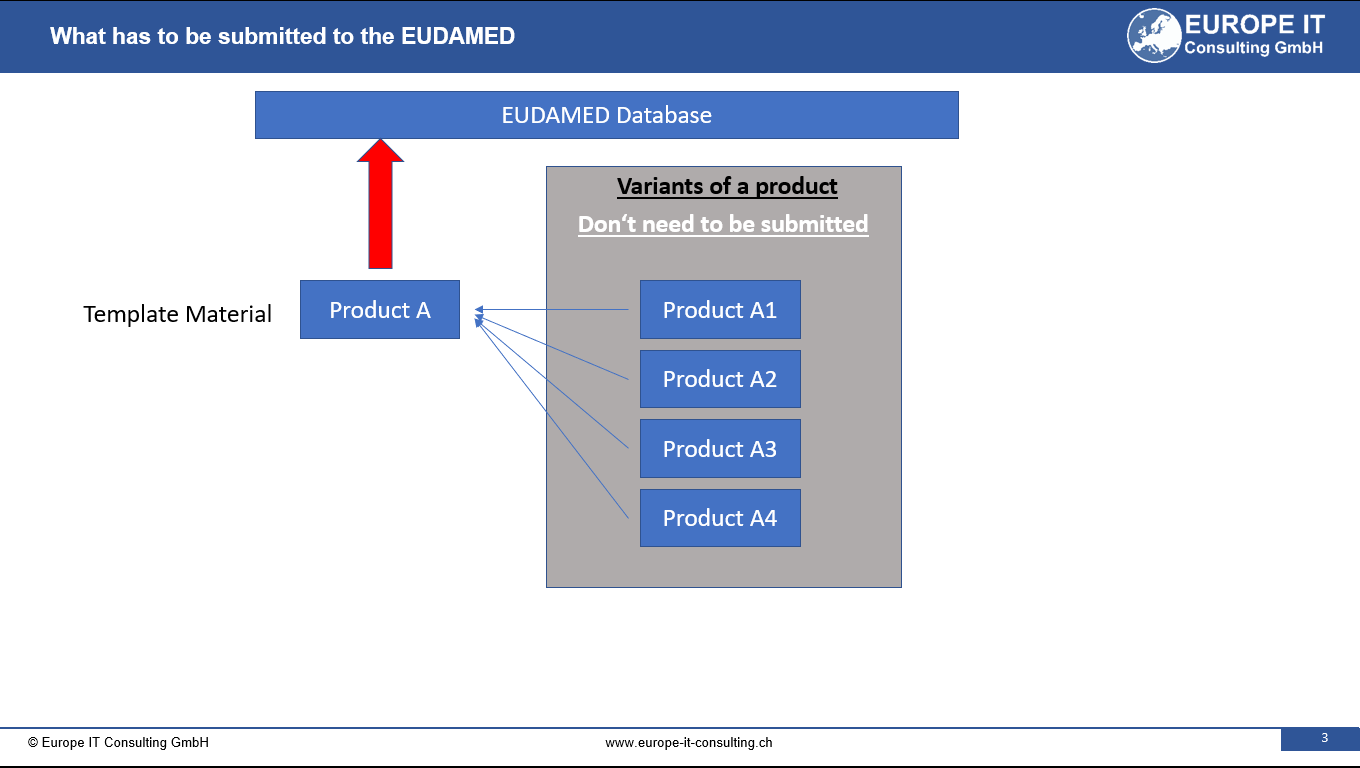


Related Posts