
Did you heard about “EUDAMED Master UDI-DI” ?
Here are the latest news about it and why the Master UDI-DI is needed.
The European Commission is amending Regulation (EU) 2017/745 to improve the Unique Device Identification (UDI) system for contact lenses and in the later stage for products which can have many variations.
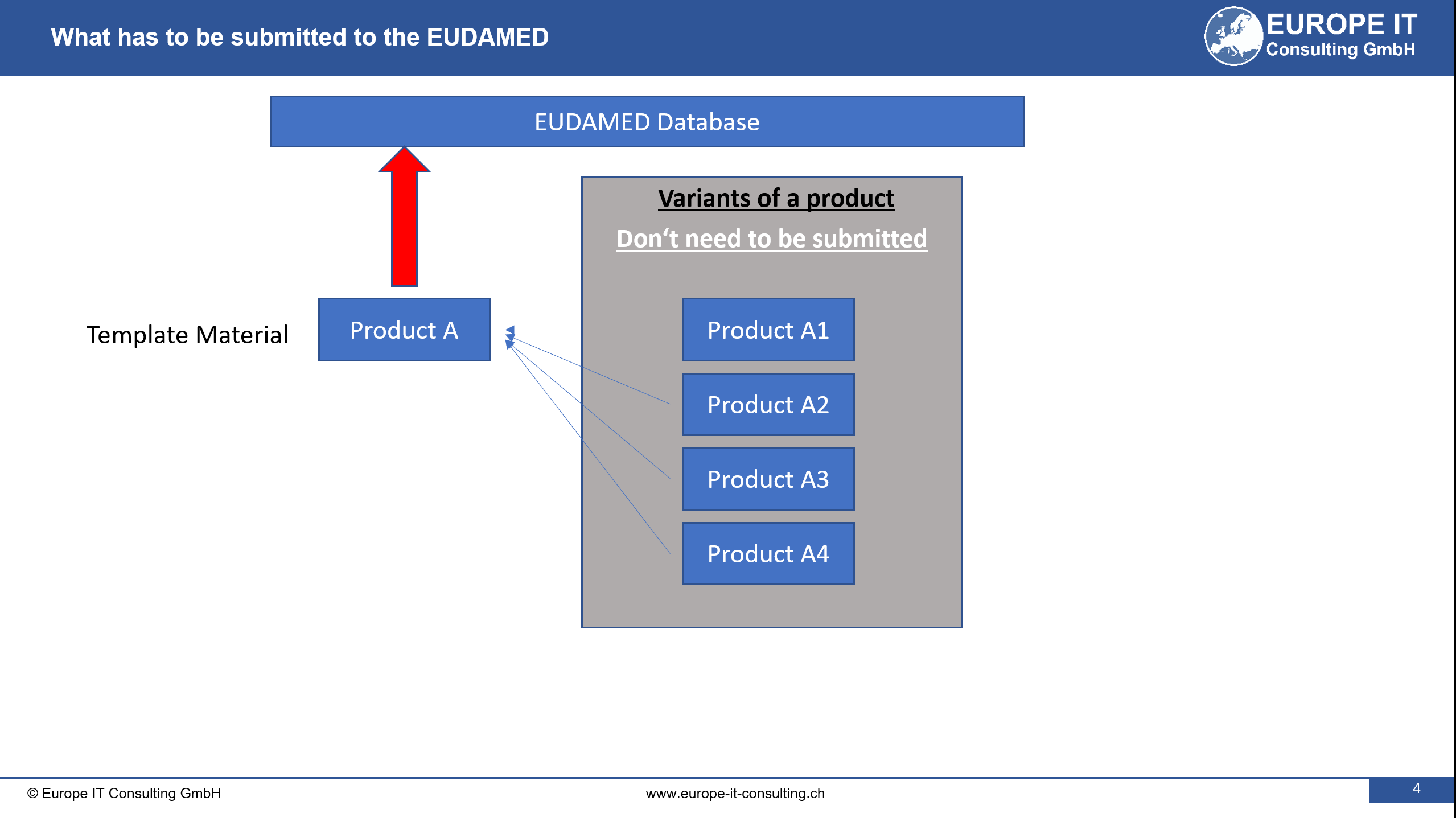
The current system requires manufacturers to assign a UDI to each variant of contact lenses, which has led to an overwhelming number of UDI-DIs (Device Identifiers) in the European Database on Medical Devices (Eudamed).
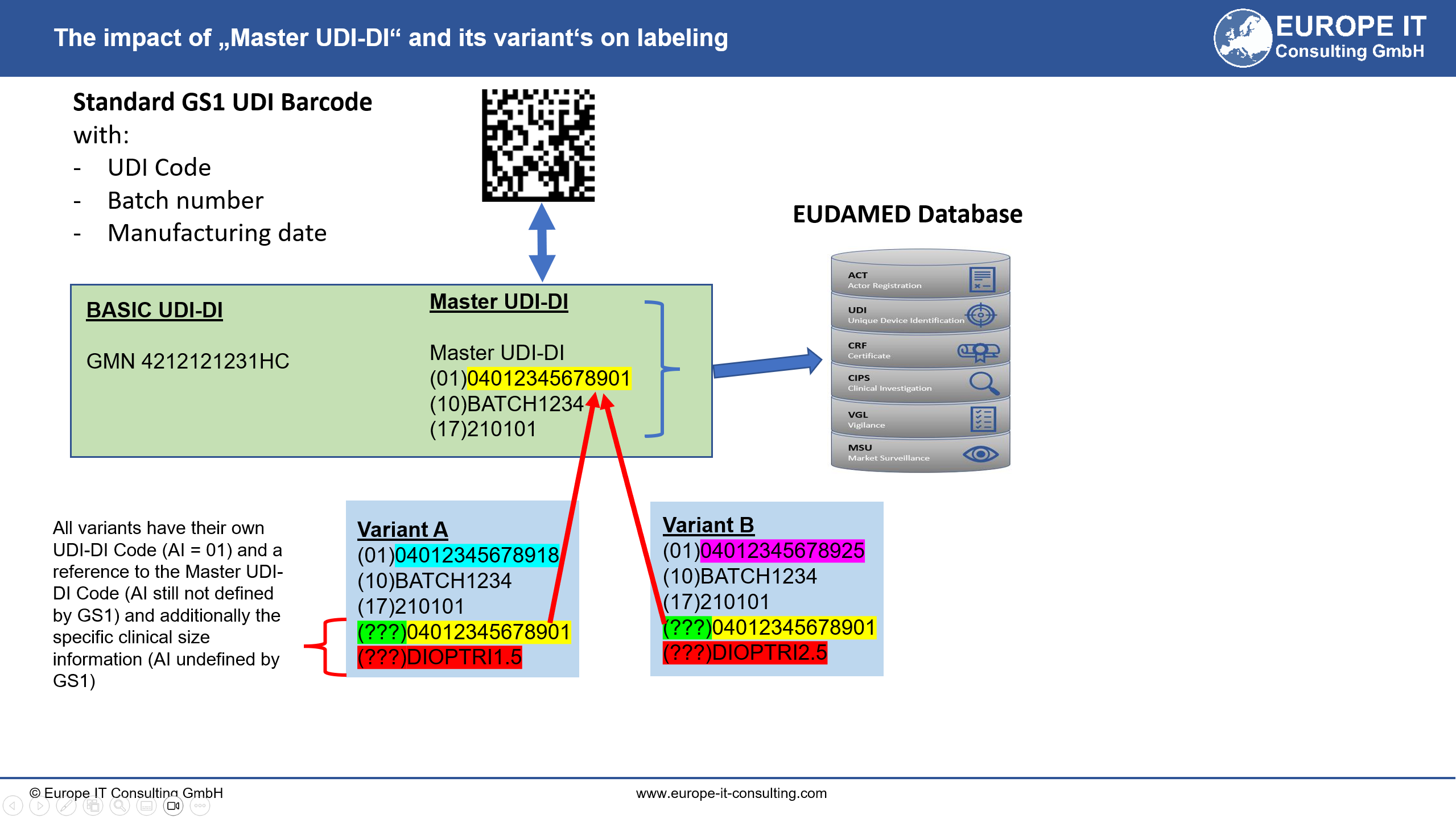
To address this issue, the Commission has developed the “Master UDI-DI” concept, which groups contact lenses with the same clinical and design parameter combinations under one identifier. This amendment will make the identification process more efficient, without compromising safety or traceability.
The new regulation will apply two years after its entry into force, but manufacturers may start using the Master UDI-DI and UDI-PI (Production Identifier) earlier if desired.
Download Link to EUDAMED Master UDI-DI draft file: EC Master UDI Draft – Europe IT







Related Posts