The introduction of the Medical Device Regulation brought a significant change: the assignment of Unique Device Identifiers (UDIs) for medical devices.
This step was taken to improve the identification, traceability and monitoring of medical devices while counteracting counterfeiting. An important milestone on this path is the implementation of the so-called Master UDI-DI.
What is the Master UDI-DI ?
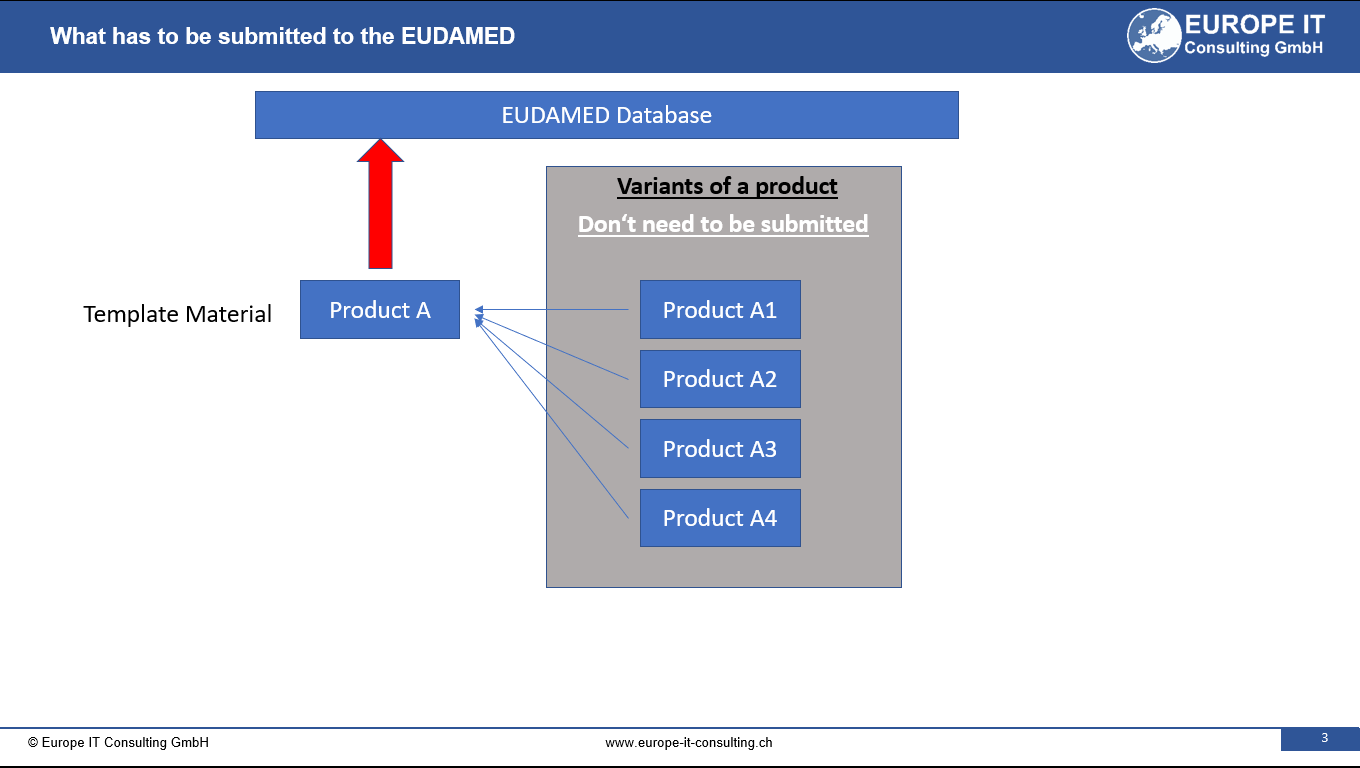
Its main objective is to provide an additional level of identification for specific products and medical devices. This innovative approach enables a more efficient grouping of highly individualized devices and thereby reduces the amount of data entries in the EUDAMED database (UDI module).
The main business challenge of the Master UDI-DI is the effective categorization of highly individualized devices. This approach not only results in a reduced amount of data entry in the EUDAMED database, but also supports the provision of more detailed product information using additional application identifiers for clinical specifications.
Implementation and benefit
The Master UDI-DI is placed as a product identifier (PI) in the UDI barcode on the packaging or label of the product. The precise specifications and requirements for the Master UDI-DI may not have been finalized by EUDAMED (European Database for Medical Devices) at this time.
The first product categories requiring the Master UDI-DI include contact lenses, ophthalmic lenses, and finished lens devices. Nevertheless, it is important to emphasize that the list of products with a high degree of customization could be expanded in the future.

The implementation of the Master UDI-DI may seem like an additional hurdle, but it plays an essential role in the process of medical device identification and traceability.
This measure underlines the continuous strive for higher safety, quality and efficiency in the field of medical devices.










Related Posts