
UDI Compliance & Software Validation (CSV): Challenges, Solutions, and Future Perspectives on UDI
With the introduction of the UDI system in Europe, the USA, and a growing number of other countries, manufacturers are now obligated to comply with Unique Device Identification regulations. However, these obligations pose a challenge for many companies, as the path to compliance is extremely complex.
Ensuring future compliance in the UDI space is crucial. As each medical device must be labeled with a unique identifier, the capture, management, and regular updating of UDI data in internal systems and regulatory databases are essential for every medical device manufacturer.
UDI requirements vary from one authority to another since there is no global regulatory authority. While the IMDRF (International Medical Device Regulators Forum) has provided a global guideline, it has been interpreted and implemented differently by various country authorities in reality.
As a result, managing product labeling and UDI data registration in relevant authority databases such as the FDA for the USA and EUDAMED for the EC, SFDA for Saudi Arabia, etc., becomes a new challenge each time.
To not only become UDI-compliant but also to remain compliant, investments in new technologies and software solutions are unavoidable.
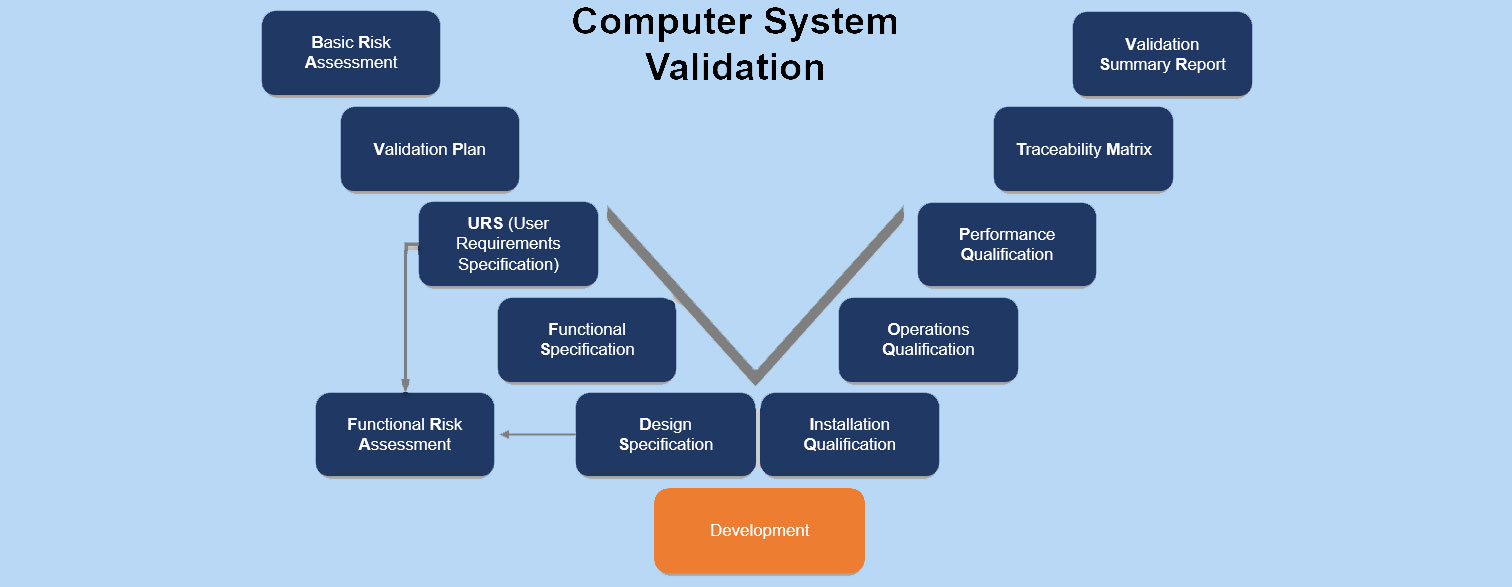
Read more about Computer System Validation (CSV) in our upcoming article.
UDI Audit and UDI Validation: Europe IT’s Global Solution
Europe IT Consulting is a leading company in the field of information technology and consulting services for the medical devices industry. Our comprehensive solutions focus on Unique Device Identification (UDI) and its associated requirements. Our goal is to support MedTech companies in providing and transferring data required by authorities to simplify the process and interaction between their ERP system and authorities, ensuring regulatory compliance.
What is an “Audit Trail” in Software?
Definition and Significance
An Audit Trail, or traceability, is a crucial element in modern software applications, especially in areas where data security and compliance are of utmost importance. It serves as a chronological record of all activities and changes made within a software application, essential for tracking and analyzing how data has been handled, modified, or transferred.
Operation of an Audit Trail
The Audit Trail logs in detail who performed an action, what the action involved, when it occurred, and in some cases, why it was performed. This information can be crucial to ensuring data integrity and security, allowing organizations to demonstrate compliance with various regulatory standards.
Key Benefits of the Audit Trail
1. Enhanced Security:
By tracking user activities, the Audit Trail helps identify unauthorized or suspicious actions, contributing to increased data security.
2. Compliance:
In many industries, companies are legally required to maintain a detailed Audit Trail to comply with standards such as GDPR (General Data Protection Regulation), HIPAA (Health Insurance Portability and Accountability) oder SOX (Sarbanes-Oxley Act) zu erfüllen.
3. Troubleshooting and Maintenance:
Audit Trails facilitate understanding issues and inconsistencies in data or system functionalities, leading to more efficient maintenance and troubleshooting processes.
4. Transparency and Accountability:
They create an environment of transparency and accountability by ensuring all actions are traceable and accountable.
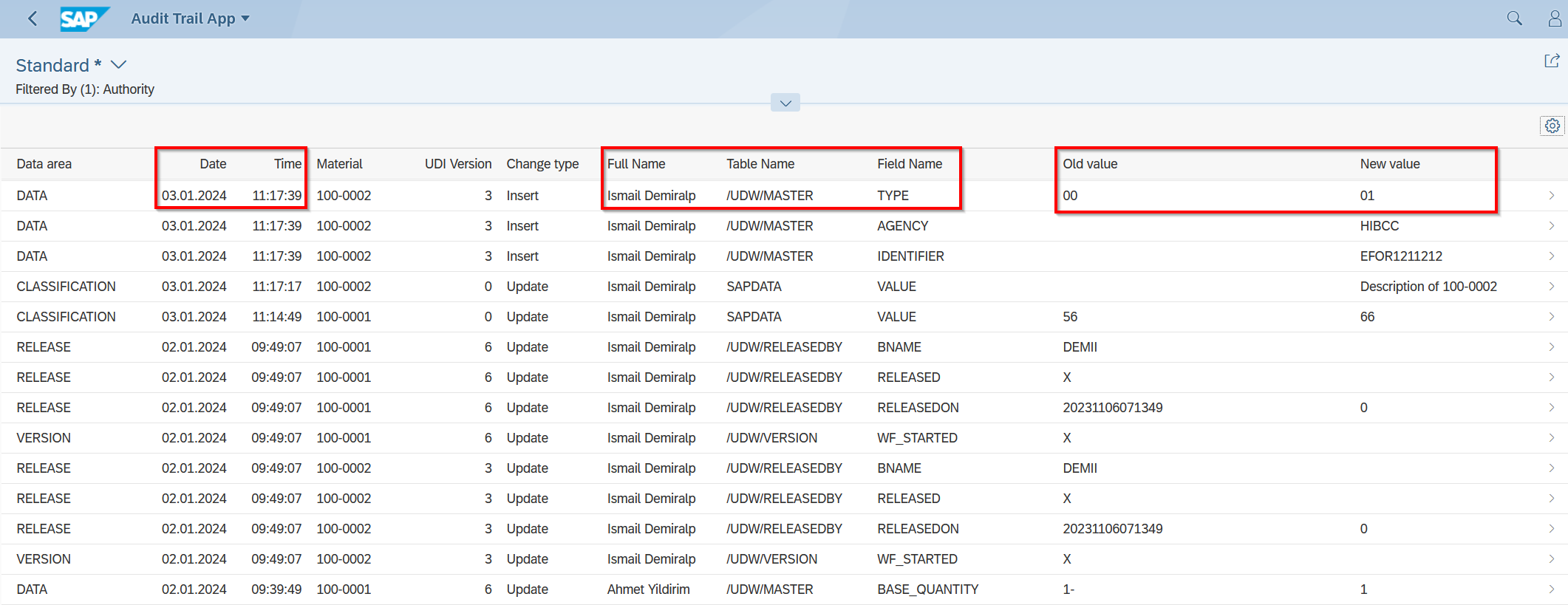
Audit Trail in UDI Software
Implementing an Effective Audit Trail
To implement an effective Audit Trail, the software should be able to automatically capture and store relevant data securely. It is essential that this data be stored securely and protected from unauthorized access. Additionally, the data should be organized to be easily searchable and analyzable.
Conclusion
The Audit Trail is an indispensable tool for companies to ensure the security and integrity of their data and meet regulatory requirements. By providing detailed records of all system activities, it enables effective monitoring, analysis, and tracking of data movements and changes.
You can check explanations for glossary terms here.
Global UDI Solution
Our Global UDI Solution can help you overcome these challenges while optimizing your business processes. Benefits include:
- Compliance with regulatory requirements in various countries
- Improvement of patient safety through clear device identification
- Enhancement of efficiency in the supply chain through simplified identification
- Improvement of data management through a unified method for capturing and managing information and documentation.
If you are interested, contact us today to learn more about our UDI solutions and MedTech consulting services and how we can help you achieve your goals.