UDI format for GS1 & HIBC
The format of your UDI codes, Basic UDI-DI and UDI-DI, depends on the selected Issuing Agency. For the two most commonly used Issuing Agencies, GS1 and HIBC, we have summarized…
The format of your UDI codes, Basic UDI-DI and UDI-DI, depends on the selected Issuing Agency. For the two most commonly used Issuing Agencies, GS1 and HIBC, we have summarized…

On the 8th February 2021 the European Commission published a Guidance on the Management of the Legacy Devices. It explains in detail how Legacy Devices will be identified and the…
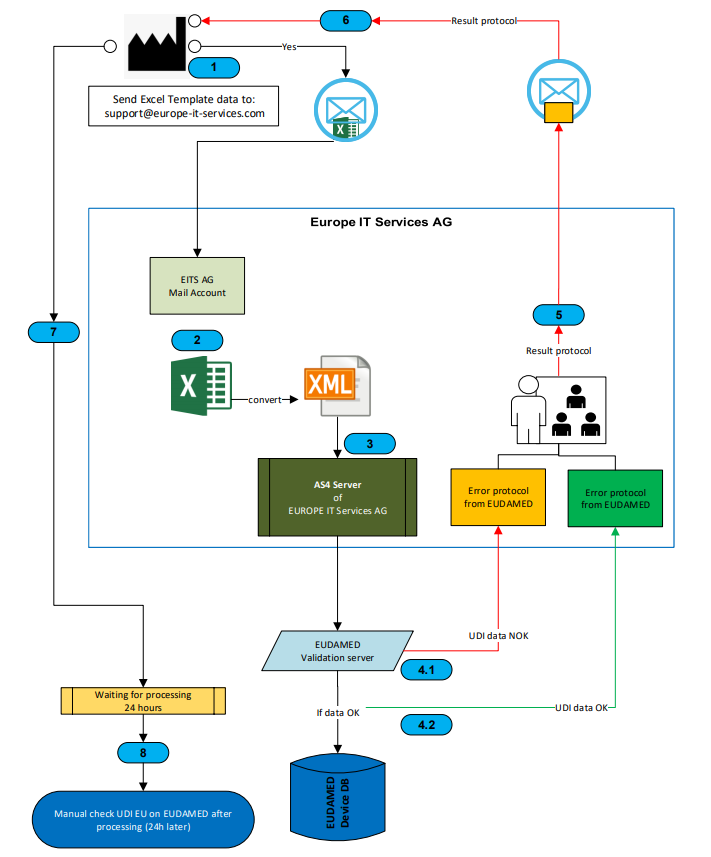
By using the Europe IT submission method, you can easily submit your UDI data to EUDAMED via Excel. But before starting to consider the data transfer process, make sure you…

Many economic operators have questions in the course of their UDI implementation, which are also asked by other economic operators. We have summarized and answered the most frequently asked questions…

You may have noticed that the UDI rules are quite complex and involve different actors.If you are in the MedTech industry, it is even more important to be clear about…

Find out here about the latest UDI Implementation Timeline for EUDAMED.
https://www.youtube.com/watch?v=vuK0Fj55x-U&feature=youtu.beWhat is UDI? What are the goals? Who is affected? How can UDI be successfully implemented?Learn all important information about Unique Device Identification for EUDAMED and FDA in this short…
What is MDR/IVDRLegislationThe new European Medical Device Regulation (MDR 2017/745) and the In Vitro Diagnostic Regulation (IVDR 2017/ 746) replace the existing medical device directives.Since 25.05.2017, the EU regulations, the…

GUDID / EUDAMEDWhat already is true for food today is also becoming reality for medical products. Traceability must be ensured, with the overriding goal of optimizing patient safety. Preparations at…

The FDA has laid the foundation with its UDI system and the GUDID database. Many countries are not yet ready to allow medical device manufacturers to automatically upload their data.…