MDR entry into force: situation in Switzerland

The Medical Device Regulation (MDR) is about to apply in the European Union, starting on 26 May 2021. Since Switzerland is not a member state, this regulation does not apply…

The Medical Device Regulation (MDR) is about to apply in the European Union, starting on 26 May 2021. Since Switzerland is not a member state, this regulation does not apply…

Information on the maximum number of reuses of devices and their implications on UDI for MDR 2017/745 compliance was recently provided by the MDCG in the Guidance on BASIC UDI-DI…

The IVDR provisions must be applied before May 26, 2022 (during the transition period). As obscurity still remains, the Medical Device Coordination Group (MDCG) recently published indications on how to deal…
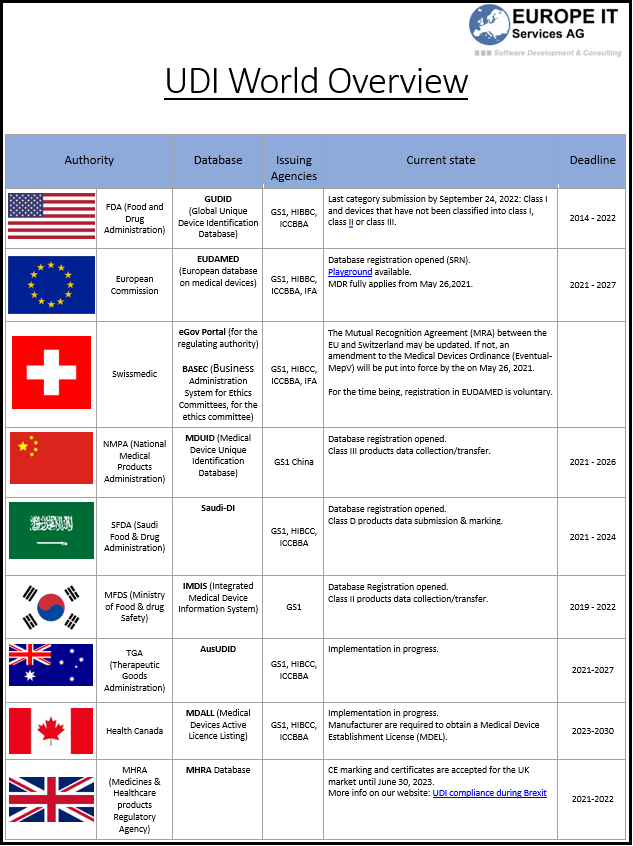
The UDI (Unique Device Identification) field is still evolving and is more or less advanced depending on the country. If you are in any way related to the medical devices…
The variety of medical devices is also expressed in their different forms. These are an important factor influencing the legal status and the way in which the medical device is…

The UK's exit from the European Union has a great impact on many companies and the medical device business. The following article summarizes the upcoming changes and explains what to…

The European Medical Devices Database (EUDAMED) is not expected to be operational and fully functional with all its 6 modules before May 2022. Nevertheless, the effective date of the Medical…
The format of your UDI codes, Basic UDI-DI and UDI-DI, depends on the selected Issuing Agency. For the two most commonly used Issuing Agencies, GS1 and HIBC, we have summarized…

Compared to other European countries, many German economic operators have not yet been assigned their Single Registration Number (SRN), and there are reasons for this. The Federal Ministry of Health…
