EUDAMED: Updated Rollout Plan for 2025-2027 Released
The European Commission has released an updated EUDAMED rollout plan, introducing changes to the implementation timeline and transition periods. Companies in the MedTech industry must take note of these updates to ensure compliance with UDI and Vigilance reporting requirements.
🔗 Official Source: EUDAMED Roadmap (European Commission)
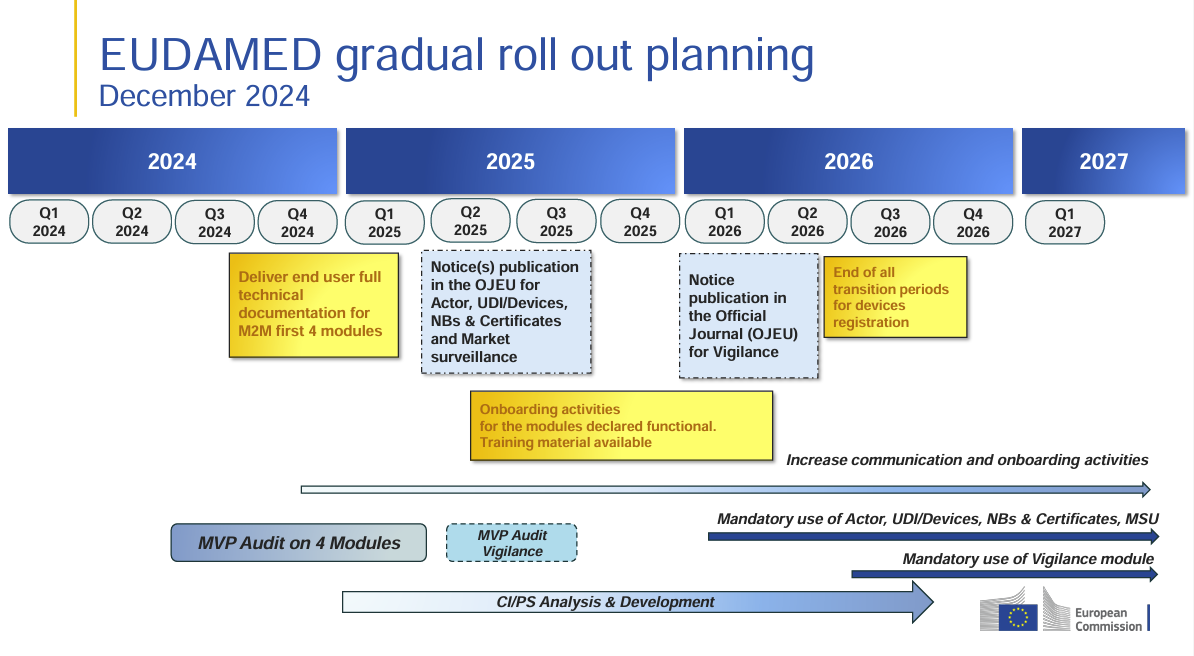
Key Changes in the EUDAMED Rollout (As of December 2024)
🔹 Q4 2024: Release of full technical documentation for M2M modules
- July 2024 version: Covered 5 modules
- December 2024 version: Reduced to 4 modules
🔹 Q2 2025: Publication in the Official Journal (OJEU) for key modules
- No changes – still applies to Actors, UDI/Devices, Notified Bodies & Certificates, and Market Surveillance (MSU)
🔹 Q3 2025: Onboarding for functional modules
- July 2024 version: “CA & STK onboarding with complete training materials”
- December 2024 version: More general wording: “Onboarding activities and training materials available”
🔹 Q1 2026: Publication of the Vigilance module in OJEU
- No changes
🔹 Q3 2026: End of transition period for device registrations
- July 2024 version: “Devices are registered in EUDAMED (End of transition period for devices registration)”
- December 2024 version: “End of all transition periods for devices registration”
- Stronger emphasis on the final termination of all transition periods
Why This Matters for MedTech Companies
🔸 Mandatory use of EUDAMED modules from 2026 for UDI, Vigilance, Market Surveillance, and Certificates
🔸 Shorter preparation period for M2M integration due to a reduced number of initial modules
🔸 New training and onboarding activities to ensure a smooth transition







Related Posts