
On April 4th, the EUDAMED was updated.
With this release, some known errors have been corrected, which were reported by Europe IT Consulting GmbH, among others.
But the main innovation is the new separate data upload service for “Market Information”

MDR-Eudamed Market Information- Upload
Unfortunately, this doesn’t make it easier for Medtech companies. Instead of using the previous services with creating, changing or adding the UDI data, the market information must now be used additionally for all changes in that data area and has to be uploaded using a separate service and thus via a specially defined XML file structure.
Even before that, you thought that it couldn’t get any more complicated than that. But the European Commission doesn’t disappoint you. True to the motto “It can be always more complicated”.
The same can be expected for other parts of the UDI data. We expect that the areas “Packaging”, “Clinical Size Types”, “Certificates” and some other tabular areas will soon have to be served via additional services.
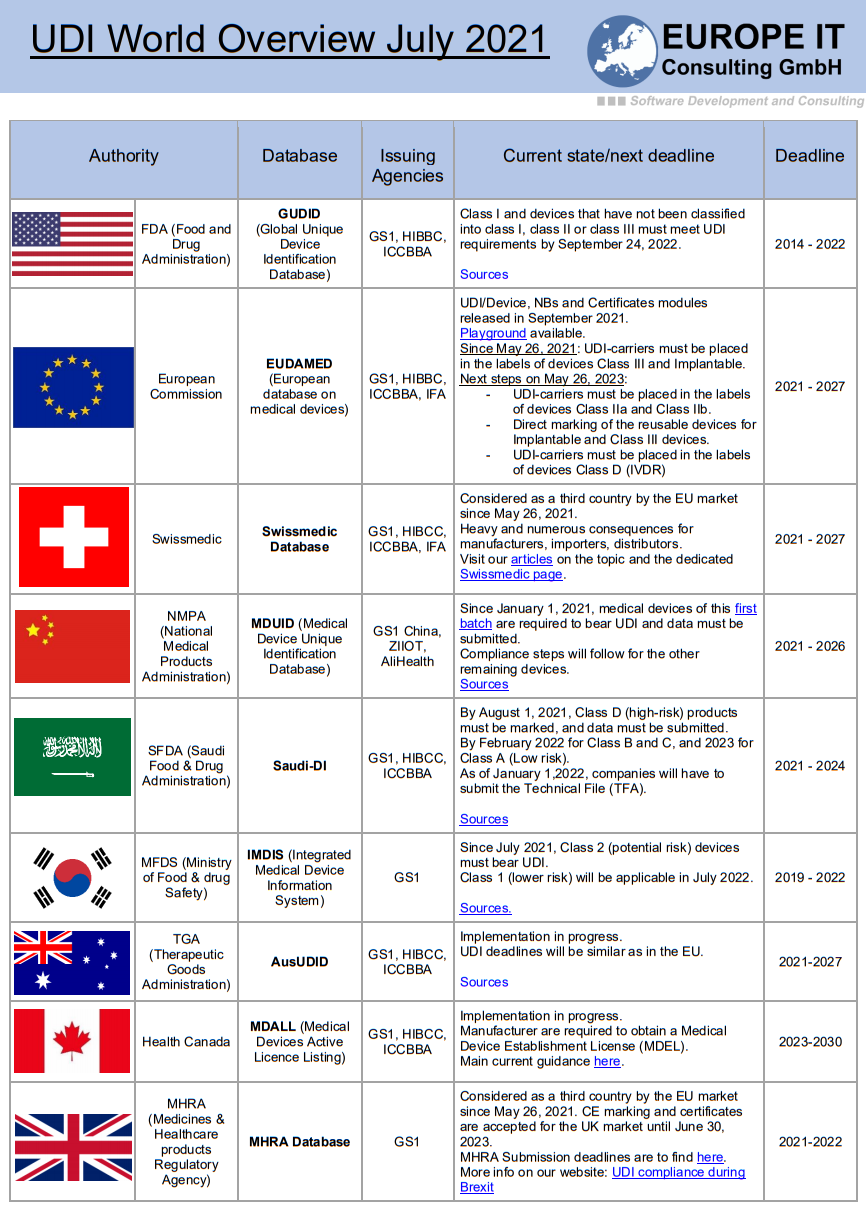
The UDI world was so easy when there was only the GUDID database 😉
Change Log of the new App version:
App-Version 2.7.0 (Last build date: 2022-04-04 07:43)
Data Dictionaries
Common
EUD Common – Data Dictionary 2.7 (no updates)
Actor
ACT – Data Dictionary 2.7 (no updates)
UDI/Device
UDI Devices – Data Dictionary 2.7 (updates from 8.1 to 2.7)
| Updated the Enum ENUM_UDID_IssuingEntity to ENUM_MDR_IssuingEntity for the Issuing Entity fields
Update the Enumeration for the Nomenclature Code field (FLD-UDID-149)- to EMDN Nomenclature Update FLD-UDID-39 (DD Legacy Devices). Update Occurrence and description |
Certificates
CRF – Certificates – Data Dictionary 2.7 updates from 7.0 to 2.7)
| Following documents to be publicly accessible:
FLD-CRF-240 Scientific opinion FLD-CRF-239 Justification (for not following the scientific opinion) |
CRF – Refused Certificates and Applications – Data Dictionary 2.7 (no updates)
CRF – Varia – Data Dictionary 2.7 (no updates)
Business rules/enums
Common
EUD Common – Enumerations 2.7 (no updates)
Actor
AIM – Enumerations 2.7 (no updates)
ACT – Business Rules 2.7 (updates)
| ocumentation version alignment, enumeration query revised |
ACT – Enumerations 2.7 (no updates)
AIM – Business Rules2.7 (updates)
| ocumentation version alignment, enumeration query revised |
UDI/Device
UDI Device – Business Rules 2.7 (updates)
| BR-UDID-113 – BR Update – Added the mention for “Providing Certificate information is optionally for MDD Class I Legacy Devices (with the exception of Measuring function ones) and for IVDD Devices having the risk class General. |
UDI Devices – Enumerations2.7 (updates)
| Update of Measure Unit enumeration – BR-UDID-800 : Clinical Size Measure Unit – ENUM_UDID_ClinicalSizeMeasureUnit (Standard MU – changed code to MU176)
Update of Enumeration for Special Device types (split by Applicable Legislation) – BR-UDID-818 : Special Device Type – ENUM_UDID_SpecialDevice |
Certificates
CRF – Business Rules 2.7 (updates)
| Added
BR-CRF-220 – Only active (not yet expired) certificates not in status WITHDRAWN can be requested for suspension/withdrawal by a Designating Authority; BR-CRF-229 – Expert panel rapporteurs are no allowed to request for change their profile; BR-CRF-233 – Mandatory to provide the validity period of the nominated expert panel list document; BR-CRF-234 – Constraints when registering a new SS(C)P version; BR-CRF-240 – Mandatory provision of comments in all certificate languages BR-CRF-241 – Mandatory to reference a Manufacturer and/or SPP Producer when registering a request for suspension/withdrawal of certificates not yet registered in EUDAMED by a Designating Authority; BR-CRF-243 – Provision of decision document when registering an application; BR-CRF-245 – Specific criteria when searching for certificates to be merged; BR-CRF-251 – Possibility to change a quality certificate scope when restricting or reissuing a quality certificate type containing devices and systems and/or procedure packs sterilisation; BR-CRF-261 – Viewing discarded requests for suspension/withdrawal of certificates; BR-CRF-262 – A DA can register requests for suspension/withdrawal of certificates only for certificates issued by Notified Bodies that are under responsibility of that DA. Changed BR-CRF-163 – Scientific opinion document will be mandatory to provide if applicable. Depending on the outcome from the screening panel if the opinion will be provided or not. When opinion will be provided, Notified Bodies must provide the scientific opinion document within the mechanism for scrutiny step of certificate registration; BR-CRF-249 – Possibility to add/remove languages when amending or reissuing a certificate. |
CRF – Enumerations 2.7 (updates)
| Added enumeration for the request for suspension/withdrawal of certificates |
Data exchange documents
Service Definition
DTX for EOs – Services Definition 2.7 (hier sind keine Updates ersichtlich im Dokument)
DTX for CAs – Services Definition 2.7 (hier sind keine Updates ersichtlich im Dokument)
DTX for NBs – Services Definition 2.7 (hier sind keine Updates ersichtlich im Dokument)
XSD
XML Samples
Please ensure to update the version in the XML sample to match the appropriate XSD version for the environment before testing uploads
DTX Notes documents
DTX for EOs – User guides 2.7 (updates)
5.1.1 UDI/Device services updates
5.1.2 XSD element changes
|
DTX for CAs – User guides 2.7 (updates)
5.1.1 UDI/Device services updates
5.1.2 XSD element changes
|
DTX for NBs – User guides 2.7 (updates)
5.1.1 UDI/Device services updates
5.1.2 XSD element changes
|







Related Posts