With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept here, and understand what this change means to you as an EUDAMED operator.
The implementation of the EMDN is made in order to facilitate the code search by operators using GMDN (Global Medical Device Nomenclature; the international standard for naming Medical Devices). Indeed, the EMDN will be mapped to the GMDN, so that the correspondance between these two can be visible to all operators. However, the basis remains the Italian CND nomenclature, which was selected in 2019 by the MDCG to serve as such for the EMDN.
Once EUDAMED goes fully online, this new nomenclature will have to be used by manufacturers when they register medical devices in the EUDAMED database. Each UDI-DI must be associated with a code.
Find here the full nomenclature.
Benefits:
Although the nomenclature may seem cumbersome to use, it is ultimately beneficial to all. For the manufacturers, the EMDN plays an essential role in the technical documentation because it provides structure and organisation. Furthermore, it largely supports patients by providing key descriptions on all devices registered in EUDAMED.
Codes construction:
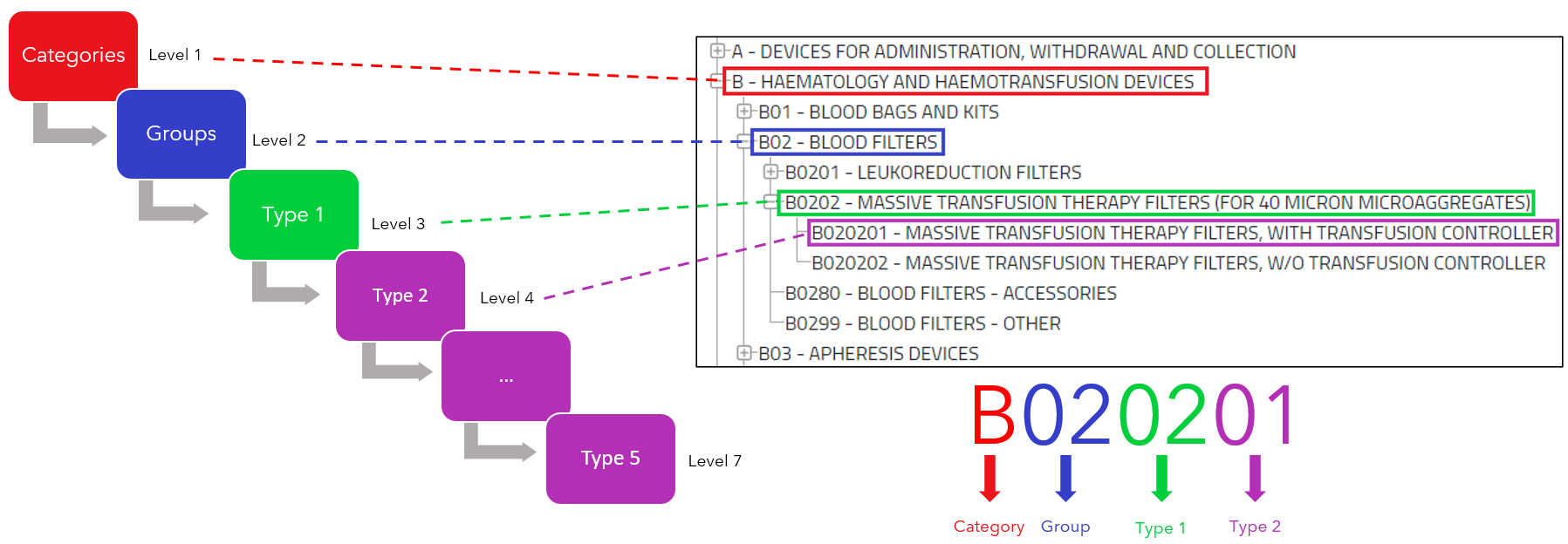
Here an example of how the EMDN codes are built:
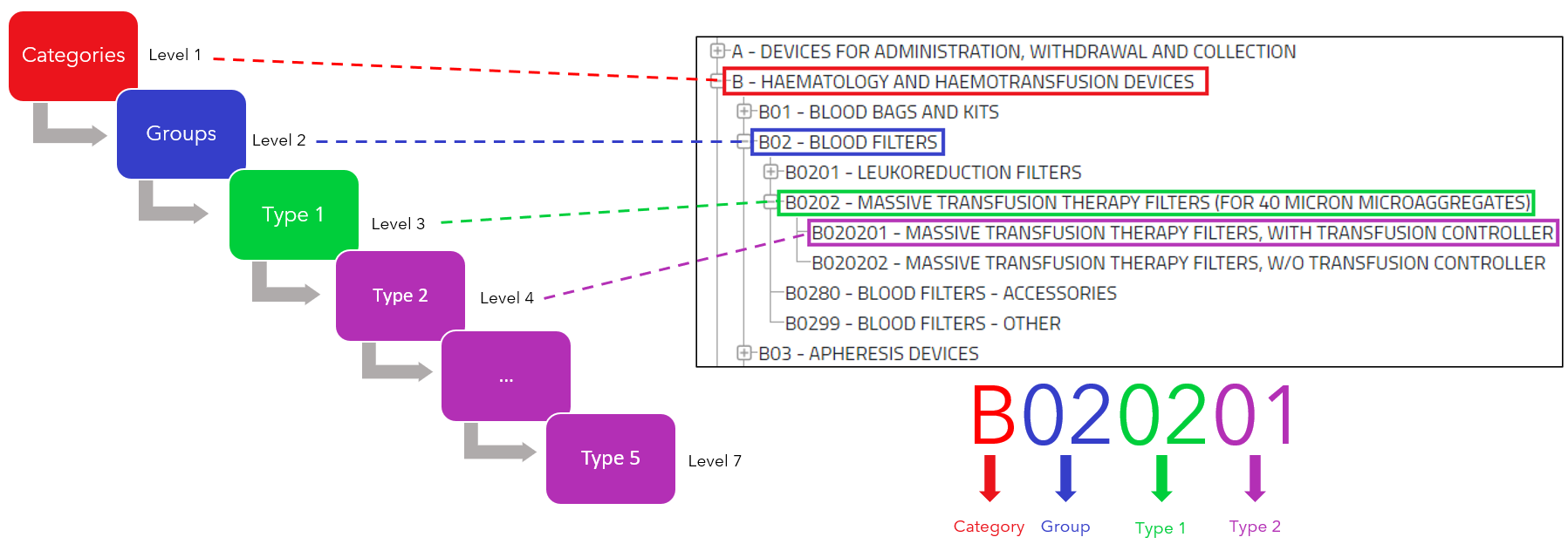
The codes construction goes from the general to the more specific.
Note that:
- “Category” ≠ “category of device”
- “Group”≠ “generic device groups”.
This tree-like hierarchy must be considered when it comes to assigning term to a device. That means the manufacturer must assign the most detailed and precised term as possible.
The European Commission is currently holding a consultation on the quality of this new nomenclature. The second version of EMDN will then be released in Q3 2021. At the same time, new terms will be added to the categories J,W and Z for the description of medical device software.
Find also here the recent MDCG FAQ on the EMDN.







Related Posts