SFDA- The Saudi Arabian authority for medical products
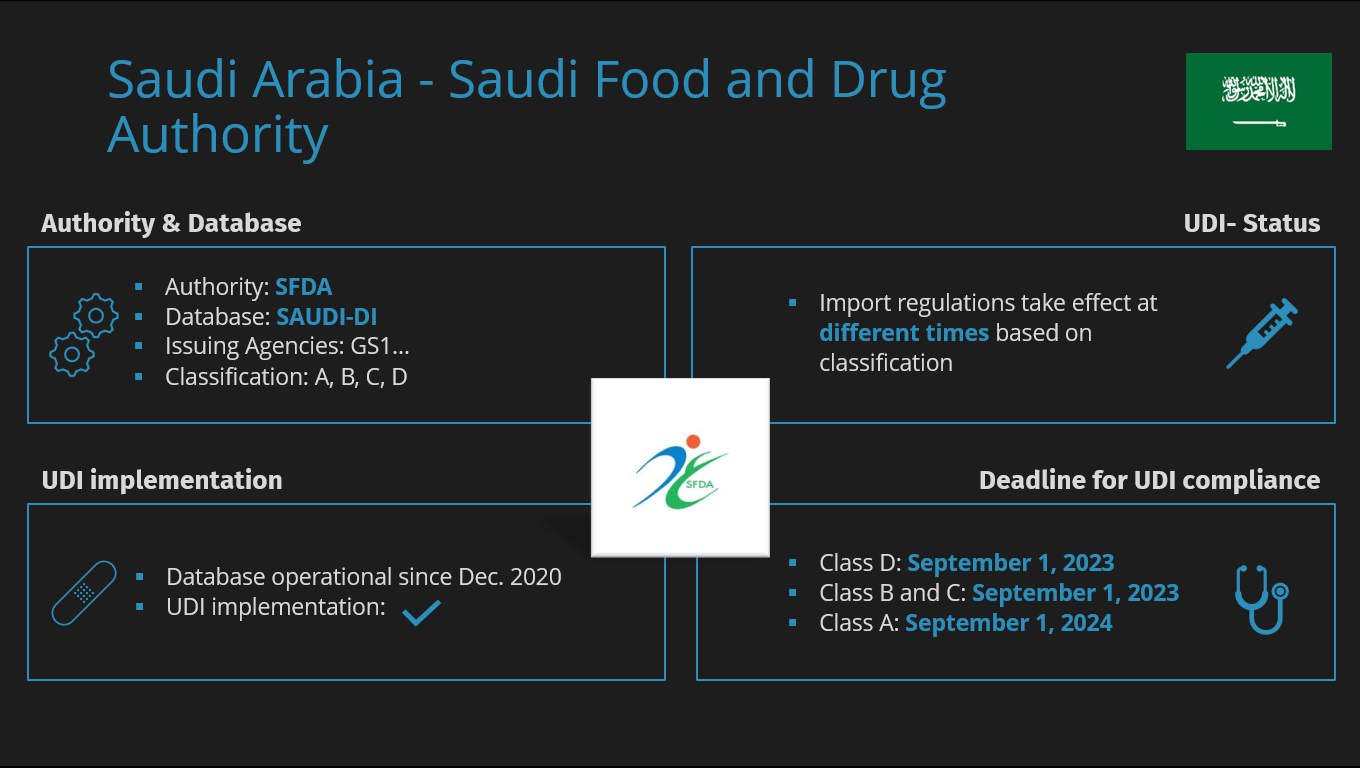
The Saudi Food and Drug Authority (SFDA) is the institution in Saudi Arabia responsible for regulating and overseeing medical devices and health products.
The primary task of the SFDA is to ensure the safety, accuracy, and effectiveness of medical devices, as it has the authority to monitor the relevant facilities and various activities throughout the entire lifecycle from manufacturing, importation to registration of products in the Saudi Arabian market.
In Saudi Arabia, medical devices are classified into 4 classes, ranging from A to D, which are categorized from low to high risk.
| Country | Authority | Classification
(low-high) |
|
 |
Saudi-Arabien | SFDA (Saudi Food and Drug Administration) | A, B, C, D |
SAUDI-DI: The SFDA Database
Like other authorities, SFDA has its own database to manage information and facilitate data transfer. The so-called SAUDI-DI aims to enable all parties to identify information about medical devices using the unique device identification code registered in the system.
|
SFDA |
|
|---|---|
| Name of the UDI database | Saudi-DI |
| Link to the UDI database | https://udi.sfda.gov.sa/ |
| Is UDI database live? | Yes |
Process for Product Registration
The 4 steps to register your products as a manufacturer:
1. Sign Up
- –> Sign up in the database and log in
2. Search
–> Search for a product using its product number
3. Data Entry
–> Enter the product-DI and provide the desired information
4. Submit
–> You can close the application and view the product information.
IMPORTANT: As a manufacturer, you must appoint an “Authorized Representative” in writing.
“Authorized Representative”:
A legal entity based in Saudi Arabia, authorized in writing by a manufacturer based outside the Kingdom, to represent them in the Kingdom for the implementation of this law and its regulations. They are responsible for compliance with product regulations, safety, post-market obligations, and maintaining the registration of medical devices.
(Source: SFDA Website)
UDI- Timeline
Although the database has been operational since December 2020, the UDI deadlines have been postponed multiple times in recent years. However, we are getting closer to the mandatory UDI submission deadline.
The extension and the last corresponding timeframe for ensuring compliance with the UDI system are as follows (As of 03.02.2024):
- Products of Classes B, C, and D must be submitted before September 1, 2023.
- Products of Class A (low-risk) must be submitted before September 1, 2024.

–> Do you want to stay updated on regulations and deadlines worldwide? Europe IT Consulting offers you a global overview:
Technical Requirements and Data Fields
| Data Fields | Description |
| Device Type | Classification of the device type based on its function or purpose of use. |
| Primary UDI-DI as labeled | The unique device identification number as labeled on the device or packaging. |
| Listing Number | A reference number identifying the device in the national or regional register. |
| Is this record for an accessory ? | Indicates whether this record is for an accessory of the main device. |
| Accessory brand name | Brand name of the accessory, if applicable. |
| Accessory model number | Model number of the accessory, if applicable. |
| Specify the model/variant | Specification of the model or variant of the device. |
| UDI DI issuing agency | The organization issuing the UDI-DIs (Device Identifier), e.g., GS1, HIBCC. |
| Quantity | The quantity of the product in the packaging. |
| Unit of Use UDI-DI | The UDI-DI for each unit of use, if applicable. |
| Production identifiers (Lot number) | Specific production information such as lot number. |
| Production identifiers (Serial number) | Specific production information such as serial number. |
| Production identifiers (Expiration (use by) date) | The expiration date of the product. |
| Production identifiers (Manufacturing date) | The manufacturing date of the product. |
| Production identifiers (Software version) | The version of the software, if applicable. |
| Is it a software? | Indicates whether the product is software. |
| Restrict number of reuses as single use device | Restriction of reusability for single-use devices. |
| Is the device considered as single item | Indicates whether the device is considered a single item. |
| Production identifiers (Expiration (use by) date) | The expiration date of the product. |
| Production identifiers (Manufacturing date) | The manufacturing date of the product. |
| Production identifiers (Software version) | The version of the software, if applicable. |
| Is it a software? | Indicates whether the product is software. |
| Restrict number of reuses as single use device | Restriction of reusability for single-use devices. |
| Is the device considered as single item | Indicates whether the device is considered a single item. |
| The device is shipped in only one level of packaging | Indicates whether the device is shipped in only one level of packaging. |
| DI for highest level (Package DI) | The UDI-DI for the highest level of packaging. |
| Package Type | The type of packaging. |
| Quantity per package | The number of units per package. |
| DI of the next lower package. | The UDI-DI for the next lower level of packaging. |
| package Dis List DI (1) | UDI-DI for the first level of packaging below the highest. |
| package Dis List Package Type (1) | Packaging type of the first level below the highest. |
| package Dis List Quantity per package (1) | Number of units per package on the first level below the highest. |
You can access the official SFDA document containing all detailed requirements via this link:
https://www.sfda.gov.sa/sites/default/files/2022-06/RequirementsUDI_0.pdf
Europe IT Consulting GmbH’s Solution
Our comprehensive Global Unique Device Identification (UDI) solution also offers effective data transmission to the SFDA. With our offering, we can help you successfully address these challenges while optimizing your business processes.
With our experience and expertise from numerous SAP development projects, we are able to find and implement an individual and optimal solution for your company.
Our solution assists you in maintaining your UDI-relevant products in SAP and transferring them to medical device databases.
For more information about UDI :
Subsribe to our monthly UDI NewsletterUDI Webinars
UDI Technical article
Are you interested?







Related Posts