
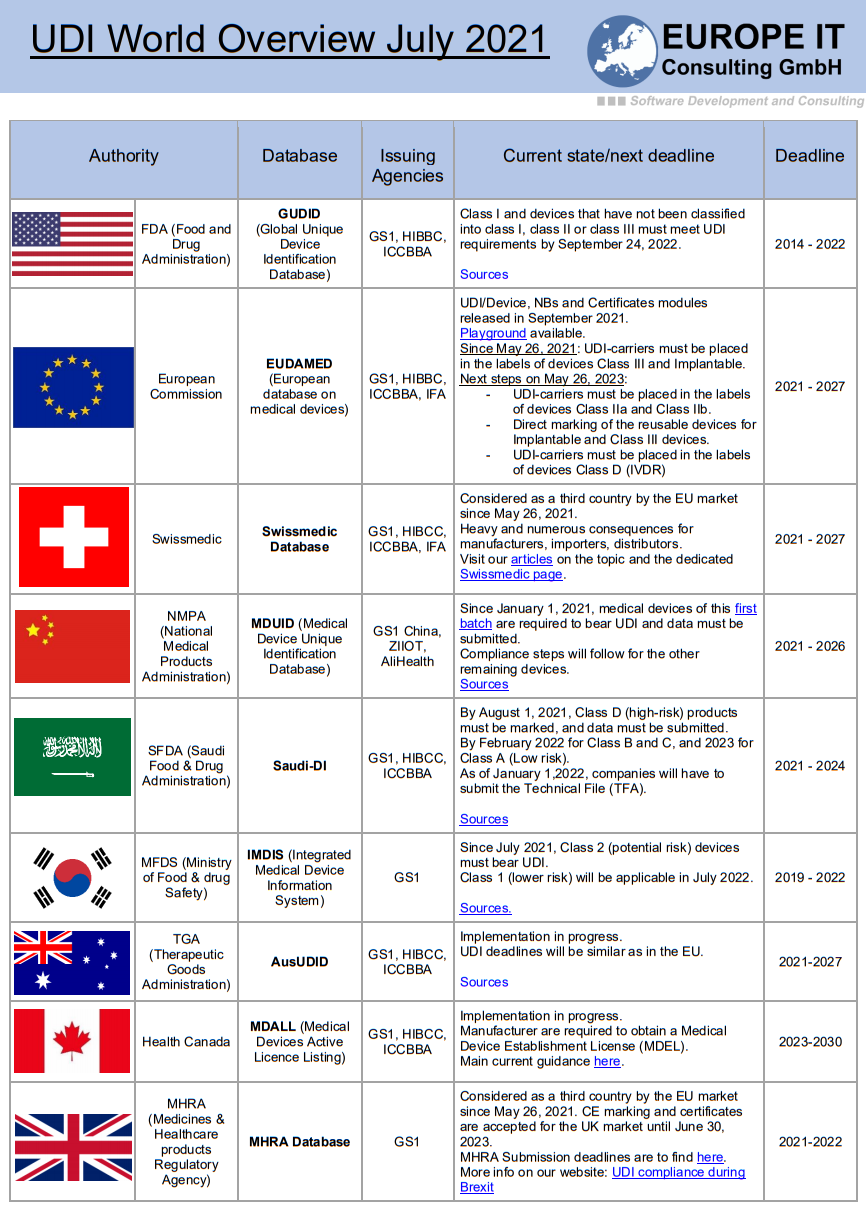
Significant EUDAMED Update: New Timeline
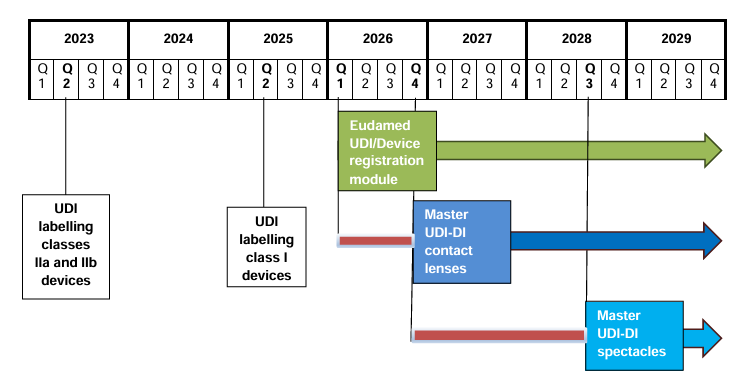
Heads up, everyone! There’s been a shift in plans. EUDAMED’s full functionality is now slated for Q2 2027.
In late October 2023, the European Commission published ‘draft’ EUDAMED timelines, marking yet another delay in its implementation. While it may seem unusual for the Commission to release a draft, they have confirmed that this delay is indeed legitimate.
The development of the Clinical Investigation and Performance Study module (CI/PS) will be temporarily halted, resuming in Q2 2024 and continuing until Q3 2026. This extension adds two more years to the project and consequently pushes back the crucial “fully functional” EUDAMED designation.
The primary speculated reason behind this delay is the need for additional development time to expand the scope of the CI/PS module. Although this is conjecture, it’s possible that we may witness a policy change or an extension in the module’s scope.
For most companies, this delay does not alter the need to upload device data to EUDAMED. Waiting could result in a more burdensome submission process, which could lead to increased costs and resource constraints. Don’t delay and make an appointment with us now, to set up our solution that will greatly facilitate the submission of your data.





Related Posts