
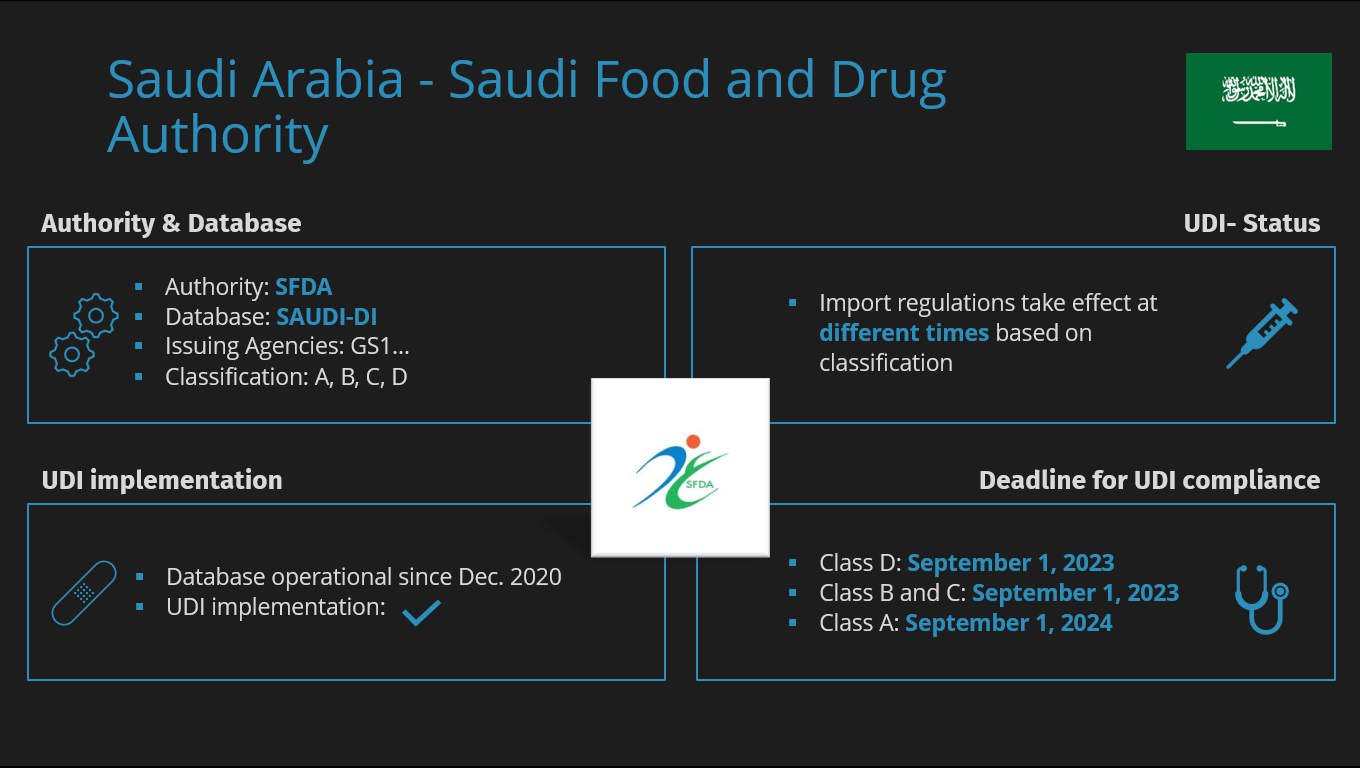
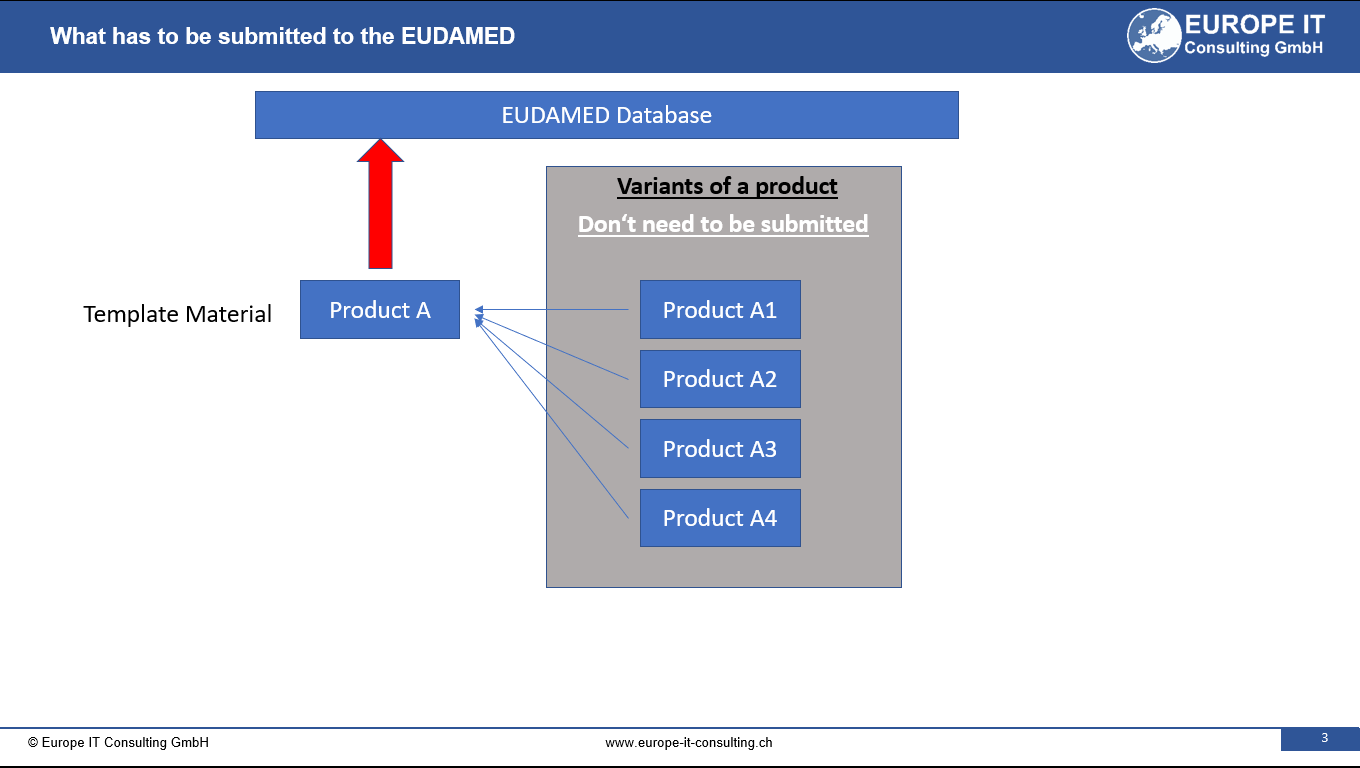
UDI SAP Add-On Release Notes 2017 After having successfully supported customers again last year with our UDI solution, we will continue to bring more customers online this year. The many positive feedbacks to our solution have encouraged us to find the right solution for our customers. to have taken the right direction. Nevertheless, we are feverishly working on further Improvements of the UDI module and the data transmission to the FDA. For customers who do not want to carry out the data transfer to the FDA themselves, we now offer the service of taking over the complete data transfer. In addition, with our “GS1 Barcode Generator for SAP” we were able to who use SAP to print your UDI-compliant labels. Today, we are already thinking intensively about how we can support the European UDI requirements can be optimally mapped with the UDI module and the UDI data already captured can be used so that you as a customer have as little effort as possible. As soon as the final data structure of the European Database for Medical Devices (EUDAMED), we will immediately start with the development work. In the new UDI Module Release 2017 several bugs were fixed, new validation rules were added and new functionalities were added. Among the new features is the mass change transaction, Extension of the “Device Description” field to 2000 characters and new print forms. For customers who are already using our UDI solution, we will be offering a new UDI solution in the next few weeks. days.








Related Posts