New nomenclature for EUDAMED: EMDN
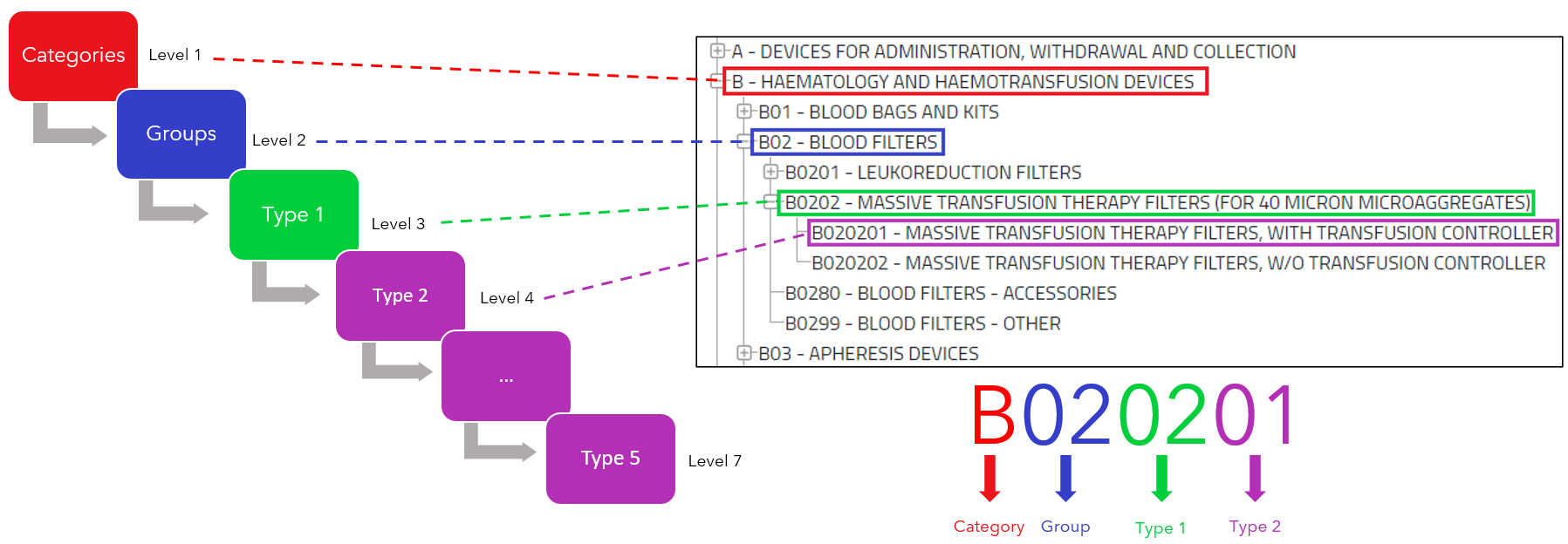
With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept…

With the implementation of the Medical Device Regulation comes the new EMDN (European Medical Device Nomenclature), as stated in the regulations (Art.26 2017/745 MDR, Art.23 2017/746 IVDR). Review this concept…

With the entry into force of MDR 2017/745 on 26.05.2021, the agreement on mutual recognition of medical devices between Switzerland and the EU lost its validity. Thus, Switzerland is now…
Find out here about the latest status (05/2021) of the UDI implementation for the EUDAMED database.
Dear Sir or Madam,You have participated in our UDI EUDAMED Webinar 2021, so you are aware of the challenges medical device manufacturers face with the Medical Device Regulation of MDR2017/745…

The Medical Device Regulation (MDR) is about to apply in the European Union, starting on 26 May 2021. Since Switzerland is not a member state, this regulation does not apply…

Information on the maximum number of reuses of devices and their implications on UDI for MDR 2017/745 compliance was recently provided by the MDCG in the Guidance on BASIC UDI-DI…

The IVDR provisions must be applied before May 26, 2022 (during the transition period). As obscurity still remains, the Medical Device Coordination Group (MDCG) recently published indications on how to deal…
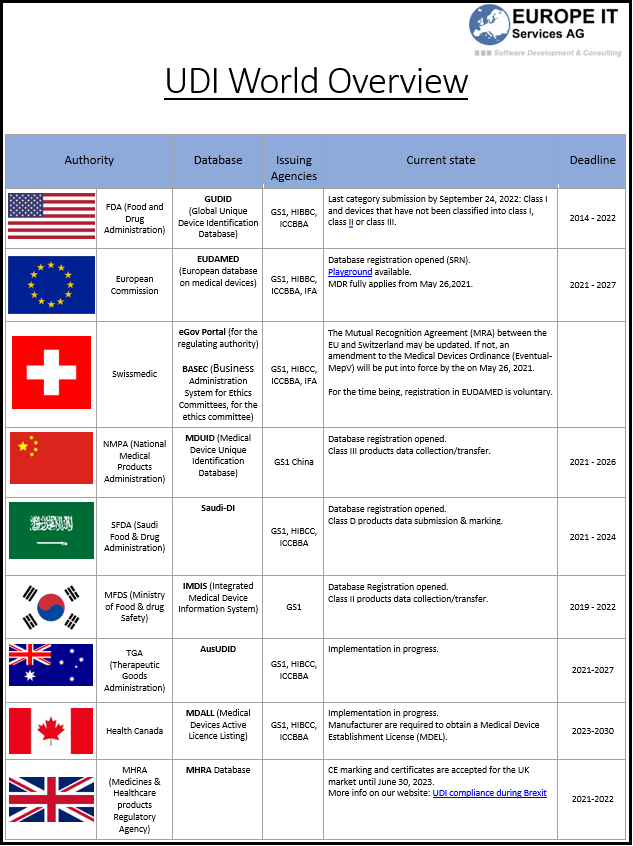
The UDI (Unique Device Identification) field is still evolving and is more or less advanced depending on the country. If you are in any way related to the medical devices…

Before jumping headfirst into the complexity of the Medical Device Regulation, you must be 100% sure that it is worth it. This assessment can be complex if you sell health-related…
The variety of medical devices is also expressed in their different forms. These are an important factor influencing the legal status and the way in which the medical device is…