Medical Devices Market in UK

Opportunities, Challenges, and Regulations The United Kingdom (UK) remains a significant market for medical devices despite its exit from the European Union (Brexit). With a growing healthcare sector, investments in…

Opportunities, Challenges, and Regulations The United Kingdom (UK) remains a significant market for medical devices despite its exit from the European Union (Brexit). With a growing healthcare sector, investments in…

Medical Mountains, the leading network for medical technology and life sciences in Tuttlingen, warmly invites you to the seminar “Implementation of the EU-MDR – EUDAMED”! This expert seminar offers in-depth…
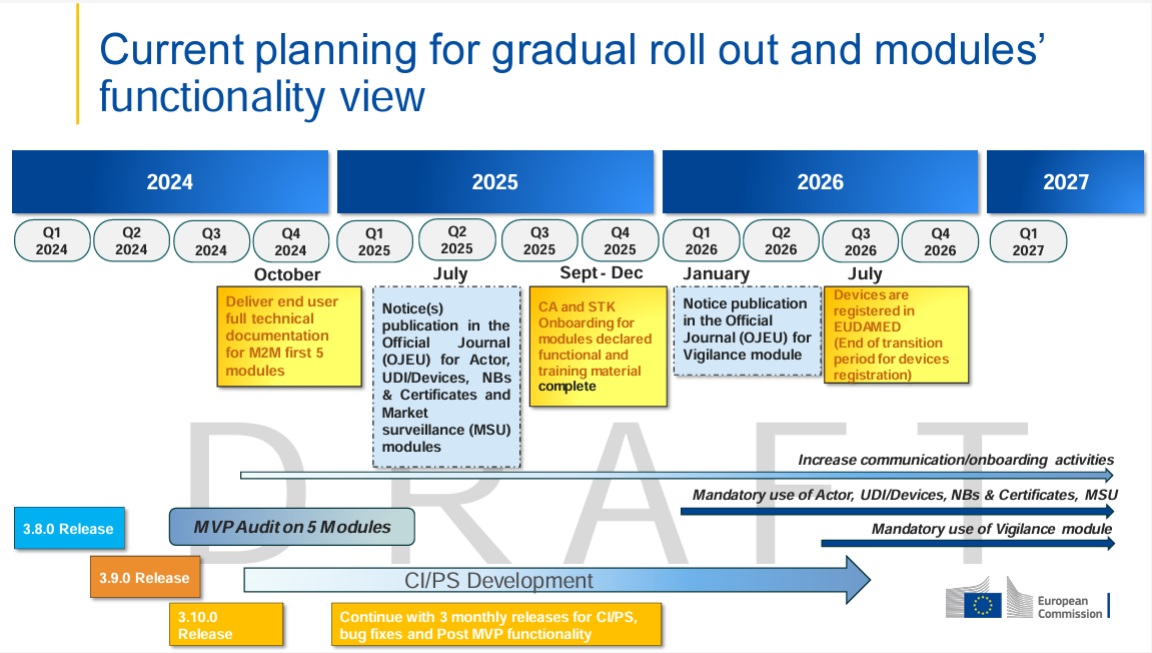
We would like to inform you today about the latest developments regarding the Vigilance module in the EUDAMED database. This crucial module, scheduled to go live in 2026, will be…

Time is running out for the medical device industry to comply with the requirements of the Medical Device Regulation (MDR). To properly implement such complex regulations, it's essential to have…