
The European Database for Medical Devices (EUDAMED) plays a crucial role in the regulation and surveillance of medical devices within the European Union. With the introduction of the regulations (Art. 26 of MDR 2017/745 and Art. 23 of IVDR 2017/746), the EMDN-codes (European Medical Device Nomenclature) have become of great importance within this system.
What are EMDN codes and why are they important?
The EMDN codes are an indispensable part of European standardization in the field of medical devices. They represent the European Medical Device Nomenclature – a uniform classification and designation of medical devices. The basis for the EMDN is the Italian CND nomenclature. When registering their medical devices in EUDAMED, manufacturers are required to specify these codes. Each UDI-DI (Unique Device Identification – Device Identifier) must be linked to a corresponding EMDN code.
The implementation of the EMDN codes aims to facilitate the search processes for users who use the GMDN (Global Medical Device Nomenclature). For this purpose, a mapping between the EMDN and GMDN codes is established to enable a clear overview for all stakeholders. In practice, these codes enable standardized and unique identification of products, which in turn greatly facilitates communication, tracking and monitoring of medical devices throughout the European market.
Finden here the complete nomenclature.
How do EMDN codes work, how are they constructed?
EMDN codes are generated based on specific criteria that take into account product characteristics, intended uses, and other relevant information. These codes consist of an alphanumeric combination and follow a hierarchical system. The length of the EMDN codes is between 3 and 14 characters, where the first character must be an uppercase letter and the following characters are numeric.
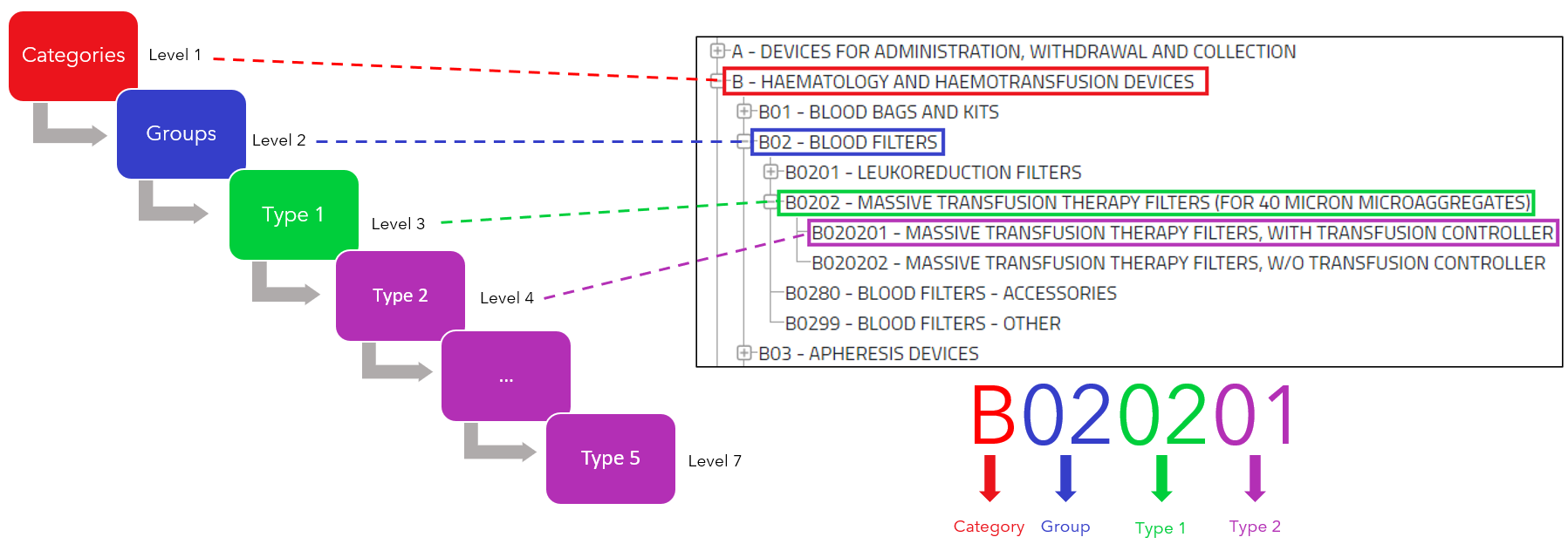
The structure of the EMDN codes follows a graded pattern leading from general to specific information. Here it is important to note :
- “category” should not be confused with “category of the device”.
- “group” should not be confused with “generic device groups”.
This hierarchy plays a central role in assigning a suitable term or code to a device. Manufacturers are therefore required to use terms or codes that are as precise and detailed as possible.
We have prepared a video that shows you exactly how to add the EMDN Codes to our SAP EUDAMED Add On
We have also prepared a 2nd video that shows you exactly how to add the EMDN codes in the EUDAMED Playground
The introduction of EMDN codes in EUDAMED marks a significant step in the regulation of medical devices within the EU.
You can also find the current MDCG FAQ on the EMDN here.








Related Posts