
SWISSDAMED – The swiss UDI database
The Mutual Recognition Agreement (MRA), one of the main agreements between the EU and Switzerland facilitating bilateral trade in a number of key sectors between the European Commission and Switzerland, has not been extended and therefore expires in March 2021.
As every medical device manufacturer in Switzerland needs an authorised representative in the EC, this has necessitated the creation of a new medical device regulation to build its own UDI database called “SWISSDAMED“.
Swissdamed – „Swiss Database on Medical Devices”
- Actor Database => Manufacturer registration to get CHRN number (comparable with SRN from EUDAMED)
- UDI Database => Contains UDI data (Structure should be similar to EUDAMED)
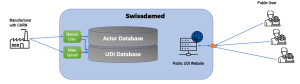
Overview of the Swissdamed Process
Registration for Swissdamed
Registered Manufacturers at Swissmedic don‘t need to register again for Swissdamed (CHRN will be assigned previously)
- Regulation EN:
- Medical Devices Ordinance (MedDO; SR 812.213)
- Vitro Diagnostic Medical Devices (IvDO; SR 812.219)
- Regulation DE:
- Medizinprodukteverordnung (MepV; SR 812.213)
- In-vitro-Diagnostika (IvDV; SR. 812.219)
- Registration form for Swissmedic / CHRN number:
- https://www.swissmedic.ch/swissmedic/en/home.webcode.html?webcode=BW630_11_001defi_FO
- To send to E-Mail: chrn@swissmedic.ch
GoLive Swissdamed planned for 2023
- The realisation will be divided into 3 phases
- Phase 1 : Registration of the manufacturers (Actor module)
- Phase 2: Registration of the UDI data in Swissdamed (UDI module)
- Phase 3: Releasing additional functionallity for existing modules

The 3 phases of the Swissdamed realization process
Homepage Swissmedic
Effects of the Medical Devices Ordinance (MepV) in Switzerland
Conformity mark and identification number
(MepV Art. 13 Conformity mark and identification number) Products that are placed on the Swiss market or made available on the Swiss market must bear a conformity mark in accordance with Annex 5.
The conformity mark listed in Annex V EU-MDR18 is also permitted as a conformity mark. In addition to the CE mark described in Annex V EU-MDR, Annex 5 of the MepV also describes a national conformity mark. Both marks are allowed.
Product Information
(MepV Art. Art. 16 product information) Product information must be in three official languages. Article 16 regulates the cases in which the product information may be written in fewer than the three official languages or in English (paragraph 3).
Implant ID
(MepV Art. 20 Information on implantable products) In addition to the product information in accordance with MepV Article 16 and Article 18 EU-MDR, the manufacturer is also obliged to enclose an implant card in the three national languages with the product.
Registration of economic operators
(MepV Art. 55 – Registration of economic operators) Manufacturers, their authorized representatives or importers register with Swissmedic within three months of placing a product on the market for the first time. Application form: Unique identification number according to Art- 55 MepV (CHRN Swiss Single Registration Number)
Medical device information system
(MepV Art. 2nd section: Information system for medical devices – Art. 83ff) Articles 83 – 92 describe an information system for medical devices. Swissmedic bears responsibility for this information system and is responsible for drawing up a user regulation. Data can be obtained from EUDAMED as well as from cantonal electronic systems.
UDI Symbols (symbol on the product or on the label)
The deadlines for UDI do not deviate from the EU-MDR and are regulated as follows (MepV Art. 104 – affixing the UDI):
- for implantable devices and Class III devices: from May 26, 2021
- for Class IIa and IIb devices: from December 31, 2027
- for Class I devices: from December 31, 2028
For reusable products where the UDI is to be placed on the product itself (Direct Mark): 2 years after the data specified for the respective product class.
Authorized Representative (MepV Art. 104a Appointment of an authorized representative)
If a manufacturer is not based in Switzerland, its products may only be placed on the market if a person based in Switzerland has been authorized.
An authorized representative must be applied within the following timeline:
- for Class III devices,
- Class IIb implantable devices and active implantable medical devices: until December 31, 2021
- for Class IIb non-implantable devices and Class IIa devices: until March 31, 2022
- for Class I devices: until July 31, 2022









Related Posts