
Identification and traceability of medical devices are crucial factors for patient safety and efficiency in the medical device industry supply chain. In this context, Unique Device Identification Barcodes play a central role.
Why are UDI Barcodes and correct UDI labeling necessary?
The introduction of UDI Barcodes and corresponding labeling is part of the UDI requirements and MDR regulation that medical device manufacturers must comply with. These regulatory requirements are especially enforced by the FDA, EUDAMED, and other global regulations.
In particular, the EU MDR 2017/745 regulation provides precise and detailed specifications for correct UDI Barcodes/labels. Understanding this is crucial to remain compliant with EU MDR.
What types of barcodes are there?
What barcode types are available for UDI?
The choice of barcode type is crucial as different barcodes support varying amounts of information and application scenarios. Here are the different barcode types that play a role in implementing UDI requirements:
- Data Matrix (Industry Standard): Data Matrix is one of the key barcode types in the medical device industry and is considered an industry standard. This two-dimensional barcode, which can be square or rectangular, is known for its high data storage capacity and robustness. Data Matrix enables the storage of extensive information in a small space, including the UDI code, batch numbers, serial numbers, expiration dates, and manufacturing dates, which is especially advantageous for small medical devices.

- QR Code: The Quick Response Code (QR Code) is another widely used barcode type known for its quick readability and large information capacity. QR codes are commonly used for marketing purposes, but they also find application in the medical device industry. They provide the ability to encode additional information such as origin, batch number, or expiration date. The QR Code is often used in the Chinese UDI system.
- Code 128: Code 128 is a linear barcode that allows high data density. This barcode type is often used in situations where a large amount of alphanumeric data needs to be encoded. Code 128 is flexible and supports various character sets, making it suitable for the identification, labeling, and transmission of information about blood products, human tissue, and organs.
Different Issuing Agencies:
In the implementation of UDI requirements, Issuing Agencies also play a crucial role. Well-known Issuing Agencies include GS1 and HIBCC. The choice of Issuing Agency directly influences the barcode type used:
- GS1: GS1 is a globally operating organization that sets standards for product identification. When GS1 codes are used, GS1 barcode types such as GS1 Data Matrix, GS1 QR Code, and GS1 Code 128 should be utilized. This ensures compliance with GS1 standards and consistent product identification.
- HIBCC: The Health Industry Business Communications Council (HIBCC) is an Issuing Agency specializing in the healthcare sector. Depending on specific requirements and agreements, the use of HIBCC codes and corresponding barcode types may be necessary.

What data must be printed on a UDI label?
The design and content of a UDI label are crucial for the unique identification and traceability of medical devices. Certain data must be accurately and legibly presented on a UDI label.
1. Symbols
A central element on a UDI label is the use of various symbols. These symbols represent information such as Manufacturer, EU Representative, Importer, Distributor, etc. Clearly depicting these symbols ensures quick and accurate data capture by scanners and other reading devices.

2. Textual Information:
In addition to symbols, textual information should also be present on the label. This can include product information such as product name, manufacturer, expiration date, and other relevant details. This information should be presented in a clear, legible font.
Example: The origin of a medical product can be labeled as follows:
Made in CC (where CC is the country code from ISO 3166-1)
3. HRI (Human Readable Interpretation):
HRI stands for Human Readable Interpretation and interprets the UDI as symbols that can be read by humans. It is the textual representation of the barcode’s content. This means that the data contained in the barcode is reproduced in human-readable form on the label. This is particularly important for users who need to manually verify information, for example, during storage, in healthcare, or when using medical devices.

Advantages of using symbols instead of text:
Using symbols instead of text offers several advantages, especially in an international context. An example is the symbol for “Single Use.” Instead of listing the text “Single Use” in different languages, a uniform symbol can be used. This not only optimizes the available space on the label but also significantly reduces the effort for multilingual labels. The consistent use of internationally recognized symbols also promotes universal understanding.
New Symbol as per ISO 15223-1:
The ISO 15223-1 is the most important of the mentioned standards and is currently in the coordination process for the 2020 version. In addition to changes to existing symbol definitions, some new symbols will be added. The specific symbols to be added will only be known after the publication. We have listed a few of the symbols expected to be included in the new standard in the following table.

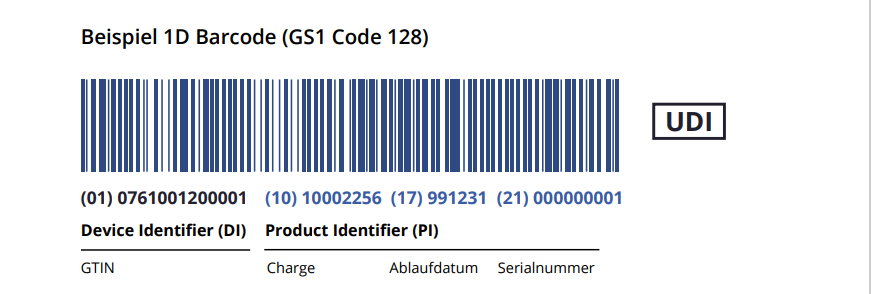
What are UDI-DI and UDI-PI? What sets them apart?
The UDI enables the worldwide unique identification and traceability of medical devices. The UDI must be printed as a barcode and in plain text. All barcode formats are allowed as long as the data structure follows the ISO 15418 standard.
The UDI number includes two components:
- UDI-DI: Device Identifier, Static Product Identifier (e.g., GTIN)
The Device Identifier (DI) is a unique code assigned to a specific medical device. This code enables the unique identification and differentiation of a specific product from other products of the same kind. It ensures that each product can be tracked individually.
- UDI-PI: Dynamic Product Identifier (e.g., Batch No., Serial No., Production Date, etc.)
The Production Identifier (PI) complements the Device Identifier and includes information about the manufacturing process and batches of the medical device. The PI varies according to the manufacturer’s requirements and can include data such as batch numbers, serial numbers, manufacturing dates, and expiration dates.

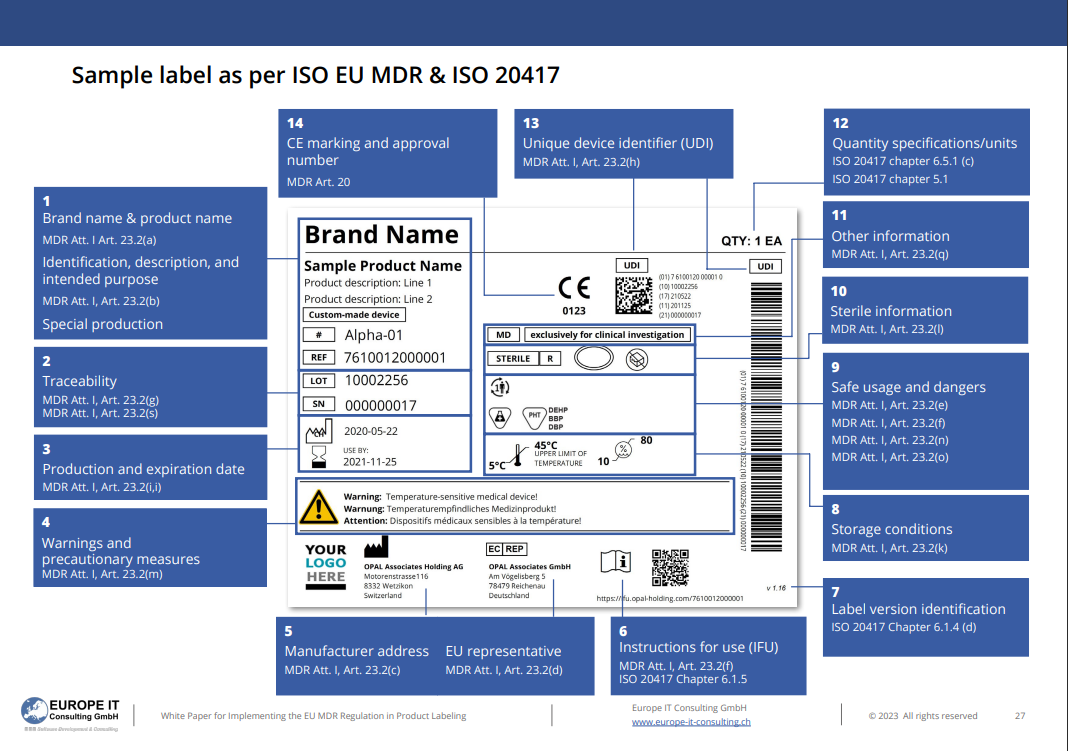
Sample of the UDI Label
Europe IT has prepared a sample for you so that you can get a concrete idea of how the label should look according to ISO EU MDR & ISO 20417.
Take our interactive quiz – in just 5 minutes!
Test your knowledge with 15 quick questions and find out where your knowledge stands on the topic of UDI Barcode and Labels. Our quiz covers various aspects, from basic principles to applicable regulations. It’s a great opportunity for industry professionals, regulatory authorities, and the curious to learn more.
Guide to Implementing EU MDR Regulation in Product Labeling
UDI labels and barcodes play a crucial role in meeting UDI requirements. Europe IT Consulting has recently released a White Paper “Guide to Implementing EU MDR Regulation in Product Labeling,” providing in-depth insights and comprehensive information. Register now to access this whitepaper:
