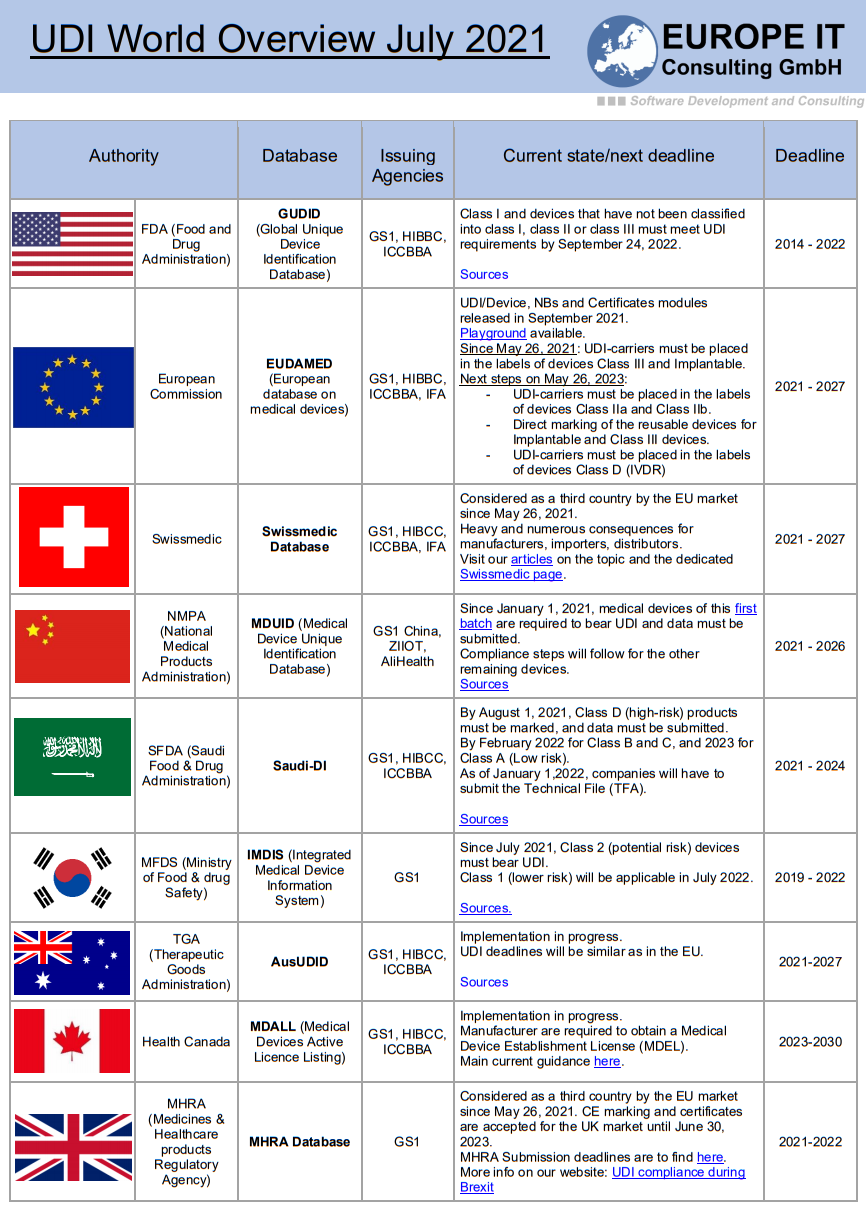
The UDI (Unique Device Identification) field is still evolving and is more or less advanced depending on the country.
If you are in any way related to the medical devices business, it may be relevant for you to stay up to date with the regulations and deadlines around the world.
That´s why Europe IT Consulting provides you with a global overview that corresponds to the status of July 2021.
Here is the PDF version.
If you want more information about the EU or FDA Medical device regulatory field, check our pages with the most important UDI links to EUDAMED or UDI Links of the FDA/GUDID.







Related Posts